Amylose is a polysaccharide made up of α-D-glucose units linked by α-(1→4) glycosidic bonds, with few branches connected to the main chain by α-(1→6) glycosidic bonds.[17]
Together with amylopectin, it is one of the two main constituents of the starch, the major storage form of energy and carbohydrates in the Biosphere.[12]
Its synthesis is catalyzed by the enzyme granule-bound starch synthase or GBSS (EC 2.4.1.242), and requires the presence of a second protein, named protein targeting to starch 1 or PTST1, with no catalytic activity.[15]
Inside the starch granule, amylose is embedded within the semi-crystalline matrix formed by amylopectin.[13] Unlike amylopectin, amylose is not necessary for the formation of starch granules, is present in smaller amounts, but it has a great influence on the physicochemical properties of the starch.[7]
Plants have been selected whose starch granules hold negligible amounts or, conversely, very high amounts of amylose. These phenotypes have both industrial applications and potential health benefits.[4][20]
Contents
- Structure
- Amylose location in the starch granules
- Synthesis
- Amylose/amylopectin ratio
- High amylose starches
- References
Structure
Amylose molecules have a molecular weight of about 106 daltons, are mostly linear and made up of α-D-glucose units, hereinafter referred to as glucose, linked by α-(1→4)-glycosidic bonds, namely, covalent bonds between C-1 of one unit and the hydroxyl group on the C-4 of the next unit.[1] The linear chains are made up of a number of monosaccharides ranging from a few hundred to several thousand; therefore, they are much longer than amylopectin chains.[17]
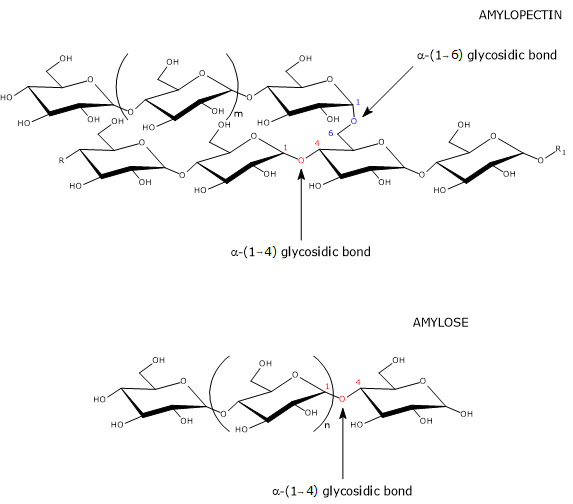 The few branches are connected to the linear chain by α-(1→6) glycosidic bonds, such as in amylopectin and glycogen, the storage form of carbohydrates in animals. An α-(1→6) glycosidic bond is a covalent bonds between C-1 of one unit and the hydroxyl group on the C-6 of another glucose unit. The number of branches is between 5 and 20, depending on the botanical origin of the starch, and the branches, compared to amylopectin, are not grouped.[6] Studies on the length of the amylose branches have shown a bimodal distribution, with the two fractions termed as:
The few branches are connected to the linear chain by α-(1→6) glycosidic bonds, such as in amylopectin and glycogen, the storage form of carbohydrates in animals. An α-(1→6) glycosidic bond is a covalent bonds between C-1 of one unit and the hydroxyl group on the C-6 of another glucose unit. The number of branches is between 5 and 20, depending on the botanical origin of the starch, and the branches, compared to amylopectin, are not grouped.[6] Studies on the length of the amylose branches have shown a bimodal distribution, with the two fractions termed as:
- AM1, which includes the shorter chains, with a degree of polymerization between 100 and 700 daltons;
- AM2, which includes the longer chains, with a degree of polymerization between 700 and 40,000 daltons.[19]
A similar bimodal distribution of the length of the branches is also observed for amylopectin, whose fractions are indicated as AP1, shorter and more abundant, and AP2.
The intraspecies variation of the distribution of the AM1 and AM2 fractions is relatively small, whereas it is large between different species, variation that has a genetic basis.[19]
Amylose location in the starch granules
The precise location of amylose in the starch granule is not known, although it is believed that most are found in the amorphous regions. However, some studies have suggested that its localization is not restricted to the amorphous regions, but is also present between amylopectin chains and on the surface of the granules.[13] Hence, amylose could have several locations within the granule.
Synthesis
In plants, the synthesis of amylose is catalyzed by GBSS, one of the six isoforms of starch synthase.[11] The enzyme, whose action is the major determinant of amylose content of starch granules, requires the presence of the PTST1 protein.[15] Amylose branching appears to be carried out by starch branching enzyme or SBE (EC 2.4.1.18).
As in the case of amylopectin synthesis, it is believed that the enzymes involved physically interact to form multienzyme complexes, which are structures able to optimize the efficiency of the process.[17]
Since amylose synthesis requires a pre-existent amylopectin matrix in order to target the granule-bound starch synthase to the starch granules, the synthesis of the two polysaccharides is not entirely simultaneous.[11]
In the initial steps, the granule-bound starch synthase, like the other starch synthases, requires short malto-oligosaccharides or MOS, α-(1→4) glucans with a degree of polymerization of 2 to 7, which act as a primer and are elongated.[17] MOS can originate from various sources, such as the trimming process of nascent amylopectin molecules by starch debranching enzyme, or from the activity of starch phosphorylase (EC 2.4.1.1), another enzyme involved in starch metabolism.[16] As MOS are poorly water soluble, they seem able to evade the hydrolytic action of alpha-amylase (EC 3.2.1.1) and beta-amylase (EC 3.2.1.2), and diffuse within the starch granule matrix where they are elongated by GBSS.[14] Once malto-oligosaccharides are elongated beyond seven glucose residues, they cannot easily diffuse out of the granule, and are further elongated.
Requiring a primer, the early stage of the synthesis of amylose and amylopectin resembles that of glycogen. However, the primer required during glycogen synthesis is the self-glucosylating protein glycogenin.
It should be noted that the polymerization of glucose to amylopectin, amylose, glycogen, and more generally, of osmotically active monosaccharides into osmotically inactive polysaccharides allows the storage of large amounts of osmotically active molecules inside the cell without an increase in osmotic pressure.
Granule-bound starch synthase
GBSS, the other isoforms of starch synthase, and starch phosphorylase belong to the glycosyltransferase family (EC 2.4).[11]
GBSS, discovered by Louis Federico Leloir’group, who had previously discovered the main pathway for the metabolism of galactose, the Leloir pathway, is the most abundant protein associated with starch granules.[9] It is present almost exclusively bound to granules, unlike the other isoforms of starch synthase which are mostly found in the plastid stroma plastids or portioned between starch granules and the stroma. Moreover, the treatment of starch granule surface with proteases showed that the majority of the granule bound starch synthase is present within the granule rather than on its surface, a location consistent with amylose synthesis within the nascent granules.[15]
In grasses, GBSS is present in two isoforms, encoded by distinct genes. The isoforms are known as GBSSI, present in the amyloplasts of storage tissues, therefore non-photosynthetic tissues, and GBSSII, present in the chloroplasts, therefore in photosynthetic tissues, where it takes part in the synthesis of transient starch.[18]
GBSS catalyzes the transfer of a glucose residue from ADP-glucose to the non-reducing end of an α-(1→4)-glucan, to which the glucose residue is joined by an α-(1→4) glycosidic bond.[5]
[(1→4)-alpha-D-glucosyl](n) + ADP-alpha-D-glucose ⇌ [(1→4)-alpha-D-glucosyl](n+1) + ADP + H(+)
Notee that starch synthases use ADP-glucose as the glucosyl donor, whereas glycogen synthase, an enzyme involved in glycogen synthesis, uses UDP-glucose.[3]
Granule-bound starch synthase is capable of adding more than one glucose monomer per substrate encounter, a processive mode of action, which differentiates it from the other isoforms of starch synthase, which are capable of adding only one glucose unit per substrate encounter, a distributive mode of action. The processive action allows the synthesis of long linear chains, and seems to be strongly increased by the presence of amylopectin.[17]
PTST1
Amylose synthesis requires the presence of a protein of the PTST family, namely PTST1, which was discovered more than fifty years after GBSS.[15]
PTST1 has no catalytic activity, but allows the binding of GBSS to the starch granule, an activity that seems more important in chloroplasts, and therefore for the synthesis of transient starch, than in amyloplasts. It was proposed that PTST1 associates with the synthase in the plastid stroma, the complex binds to the nascent starch granule, PTST1 dissociates from the enzyme which initiates the synthesis of amylose, while the PTST1 returns to the plastid stroma to recruit another GBSS molecule.
The importance of PTST1 is underlined by the fact that it is conserved throughout the plant kingdom, and its loss causes the enzyme to detach from the nascent starch granule.
Starch branching enzymes
The enzyme that generate the few α-(1→6) linkages present in amylose molecules is not known, although an isoform of the starch branching enzyme, SBEI, may be involved.[13]
SBEI is present mostly in the plastid stroma, whereas only a small proportion is bound to starch granule, and is mostly expressed in the storage tissues. The low branch frequency of the amylose could be due to its synthesis inside the granules, where SBEI is scarcely present, which would protect the nascent molecule from the action of the enzyme.[8]
Amylose/amylopectin ratio
In the starch granules of land plants, amylose is almost always present, in variable percentages, generally between 5-35%.[5] The variability occurs not only between different species, but also within the same species based on the organ or tissue considered, and, in tubers and seeds, based on the stage of development, being the content generally low in the early stages, then increasing until the final value is reached, a pattern consistent with the synthesis of amylose within an amylopectin matrix.[13]
However, there are plants whose amylose content is very low, or even absent. Their starch is referred to as waxy, due to the appearance of the endosperm of the raw grains which resembles wax. Conversely, there are plants with starch granules containing mostly, or entirely, amylose.[20]
Although its role has not yet been clarified, its near-constant presence seems to indicate this polysaccharide plays an important structural role in the starch granule, and provides the plant some advantage in the growth and development phase.[13]
The amylose/amylopectin ratio strongly influences the physicochemical properties of starch, such as its ability to absorb water, which influences processes such as starch retrogradation and gelatinization, or its resistance to enzymatic hydrolysis, which determinates, for example, the rate at which maltose and maltotriose are released during starch degradation by alpha-amylase or beta-amylase.[10] These properties, in turns, are able to affect the industrial applications of starch as well as its effects on health.
High-amylose starches
High-amylose cereals are obtained through enhancing GBSSI gene expression, or suppressing or eliminating the genes encoding for starch branching enzyme, SSIIa, or other enzymes and proteins involved in amylopectin biosynthesis. However, at least in cereal endosperm, the most effective method is the suppression or elimination of one or more starch branching enzyme isoforms.[4][20]
High amylose starches have peculiar physicochemical properties, such as a high gelling strength, an ease retrogradation, and an excellent ability to form films, properties which make them suitable for industrial applications such as the production of biodegradable plastics, paper, and adhesives.[7][10]
High amylose starches are high in resistant starch, which is a starch that escape digestion in the small intestine by alpha-amylase, one of the hydrolases involved in carbohydrate digestion. Studies conducted using foods enriched with resistant starch have shown an improvement in insulin and glycemic responses, as well as a reduction in the risk of developing cardiovascular disease, obesity, and type II diabetes mellitus.[2]
How do they work?
Resistant starch, being able to escape intestinal digestion, may low the glycemic index of the foods in which it is found, thus contributing to the regulation of blood glucose levels. Furthermore, once reached the colon, it can be fermented by the bacteria of the gut microbiota, which is part of the human microbiota, with production of short-chain fatty acids, mainly butyric acid, acetic acid, and propionic acid, fatty acids essential for intestinal health.
References
- ^ Bertoft E. Understanding starch structure: recent progress. Agronomy 2017;7:56. doi:10.3390/agronomy7030056
- ^ Birt D.F., Boylston T., Hendrich S., Jane J.L., Hollis J., Li L., McClelland J., Moore S., Phillips G.J., Rowling M., Schalinske K., Scott M.P., Whitley E.M. Resistant starch: promise for improving human health. Adv Nutr 2013;4(6):587-601. doi:10.3945/an.113.004325
- ^ Crofts N., Abe N., Oitome N.F., Matsushima R., Hayashi M., Tetlow I.J., Emes M.J., Nakamura Y., Fujita N. Amylopectin biosynthetic enzymes from developing rice seed form enzymatically active protein complexes. J Exp Bot 2015;66(15):4469-82. doi:10.1093/jxb/erv212
- ^ a b Funnell-Harris D.L., Sattler S.E., O’Neill P.M., Eskridge K.M., and Pedersen J.F. Effect of waxy (low amylose) on fungal infection of Sorghum grain. Phytopathology 2015;105(6):716-846. doi:10.1094/PHYTO-09-14-0255-R
- ^ a b Gous P.W., Fox G.P. Review: amylopectin synthesis and hydrolysis – Understanding isoamylase and limit dextrinase and their impact on starch structure on barley (Hordeum vulgare) quality. Trends Food Sci Technol 2016;62:23-32. doi:10.1016/j.tifs.2016.11.013
- ^ Hizukuri S., Takeda Y., Yasuda M., Suzuki A. Multi-branched nature of amylose and the action of debranching enzymes. Carbohydr Res 1981;94:205-213. doi:10.1016/S0008-6215(00)80718-1
- ^ a b Jobling S. Improving starch for food and industrial applications. Curr Opin Plant Biol 2004;7(2):210-8. doi:10.1016/j.pbi.2003.12.001
- ^ Kram A.M., Oostergetel G.T., Van Bruggen E. Localization of branching enzyme in potato tuber cells with the use of immunoelectron microscopy. Plant Physiol 1993;101(1):237-243. doi:10.1104/pp.101.1.237
- ^ Leloir L.F., de Fekete M.A., Cardini C.E. Starch and oligosaccharide synthesis from uridine diphosphate glucose. J Biol Chem 1961;236:636-41. doi:10.1016/S0021-9258(18)64280-29
- ^ a b Magallanes-Cruz P.A., Flores-Silva P.C., Bello-Perez L.A. Starch structure influences its digestibility: a review. J Food Sci 2017;82(9):2016-2023. doi:10.1111/1750-3841.13809
- ^ a b c Pfister B., Zeeman S.C. Formation of starch in plant cells. Cell Mol Life Sci 2016;73(14):2781-807. doi:10.1007/s00018-016-2250-x
- ^ Qu J., Xu S., Zhang Z., Chen G., Zhong Y., Liu L., Zhang R., Xue J., Guo D. Evolutionary, structural and expression analysis of core genes involved in starch synthesis. Sci Rep 2018;8(1):12736. doi:10.1038/s41598-018-30411-y
- ^ a b c d e Seung D. Amylose in starch: towards an understanding of biosynthesis, structure and function. New Phytol 2020;228:1490-1504. doi:10.1111/nph.16858
- ^ Seung D., Boudet J., Monroe J., Schreier T.B., David L.C., Abt M., Lu K.J., Zanella M., Zeeman S.C. Homologs of PROTEIN TARGETING TO STARCH control starch granule initiation in Arabidopsis leaves. Plant Cell 2017;29(7):1657-1677. doi:10.1105/tpc.17.00222
- ^ a b c d Seung D., Soyk S., Coiro M., Maier B.A., Eicke S., Zeeman S.C. PROTEIN TARGETING TO STARCH is required for localising GRANULE-BOUND STARCH SYNTHASE to starch granules and for normal amylose synthesis in Arabidopsis. PLOS Biol 2015;13(2):e1002080. doi:10.1371/journal.pbio.1002080
- ^ Szydlowski N., Ragel P., Raynaud S., Lucas M.M., Roldán I., Montero M., Muñoz F.J., Ovecka M., Bahaji A., Planchot V., Pozueta-Romero J., D’Hulst C., Mérida A. Starch granule initiation in Arabidopsis requires the presence of either class IV or class III starch synthases. Plant Cell 2009;21(8):2443-57. doi:10.1105/tpc.109.066522
- ^ a b c d e Tetlow I.J., Bertoft E. A review of starch biosynthesis in relation to the building block-backbone model. Int J Mol Sci 2020;21(19):7011. doi:10.3390/ijms21197011
- ^ Vrinten P.L., Nakamura T. Wheat granule-bound starch synthase I and II are encoded by separate genes that are expressed in different tissues. Plant Physiol 2000;122(1):255-64. doi:10.1104/pp.122.1.255
- ^ a b Wang K., Hasjim J., Wu A.C., Henry R.J., Gilbert R.G. Variation in amylose fine structure of starches from different botanical sources. J Agric Food Chem 2014;62(19):4443-53. doi:10.1021/jf5011676
- ^ a b c Wang J., Hu P., Chen Z., Liu Q., Wei C. Progress in high-amylose cereal crops through inactivation of starch branching enzymes. Front Plant Sci 2017;8:469. doi:10.3389/fpls.2017.00469