Fatty acids (FAs) are carboxylic acids with a hydrocarbon chain of varying length. The carboxyl group and the hydrocarbon chain determine the physical and chemical properties of the molecule.
In most cases the hydrocarbon chain is unbranched and, generally, with an even number of carbon atoms. It can contain only single bonds, as in the case of saturated fatty acids, or at least one double or triple bonds, as in the case of unsaturated fatty acids.
Fatty acids are members of the class of compounds known as lipids, and can be classified on the basis of chemical and physiological criteria.
They have important functions in cells: they can be oxidized to provide energy, are components of cell membranes, and are the precursors of bioactive lipid mediators.
CONTENTS
Structure
Fatty acids are carboxylic acids with the general formula R–COOH, where R– is an hydrocarbon chain. Therefore, they are made up of only carbon, hydrogen and oxygen atoms.
A carboxylic acid is an organic compound that contains a carboxyl functional group, −COOH, in which a carbon atom is bonded to an oxygen atom by a double bond to form a carbonyl group, and to an hydroxyl group by a single bond.
The R– group is attached to the fourth bond of the carboxylic carbon. R– group of formic acid, the simplest carboxyl acid, is a hydrogen atom, whereas in the other carboxyl acids it is an hydrocarbon chain that can be:
- linear or branched;
- with carbocyclic units;
- with an even or odd number of carbon atoms;
- without double/triple bonds, therefore saturated;
- with double/triple bonds, therefore unsaturated.
FAs found in foods generally have a linear carbon chain, with an even number of carbon atoms, and a length of 4 to 24 atoms; moreover the chain can be saturated or unsaturated. For a list of the most important FAs for human nutrition see the page list of fatty acids.
Branched fatty acids are common in Gram-positive bacteria, and are present in low concentration in milk and meat lipids of ruminants, an example is phytanic acid, while they are rare in plant lipids.
Polarity
The carboxyl group is a polar acid group, whereas the hydrocarbon chain is the nonpolar region of the molecule. The hydrophobicity of the chain increases as the length increases, and this determines the degree of solubility of the fatty acid in polar and non-polar solvents.
In FAs with short carbon chain, the polarity of the carboxyl group wins over the hydrophobicity of the carbon chain, the molecule has a polar nature and is soluble in polar solvents such as water and ethanol.
Butyric acid is an example of a fatty acid soluble in polar solvents. Starting from caproic acid, whose chain is two carbons longer than that of butyric acid, the polarity gradually decreases with increasing chain length. Therefore, caprylic acid, with a 8 carbon atom chain, is less polar than capric acid, with a 10 carbon atom chain, which in turn is less polar than lauric acid, with a 12 carbon atom chain.
For chain length greater than 16-18 carbon atoms, namely, from palmitic acid and stearic acid forward, the molecules are completely insoluble in polar solvent. Therefore, arachidic acid, behenic acid and lignoceric acid, three saturated molecules with chains of 20, 22 and 24 carbon atoms, respectively, are insoluble in polar solvents.
Melting and boiling point
Like polarity, melting point and boiling point also depend on the properties of the carbon chain.
Melting and boiling points of saturated FAs increase as the molecular weight increases, and therefore of the length of the chain.
Saturated linear chains have a relatively linear configuration. This allows the molecules to pack closely together, which allows the formation of many intermolecular hydrophobic bonds that stabilize the structure in an almost crystalline form. This causes the increase in melting and boiling point.
Melting and boiling points of unsaturated FAs are lower than the corresponding saturated fatty acids. This is a consequence of differences in the geometry of the carbon chain due to the presence of double bonds.
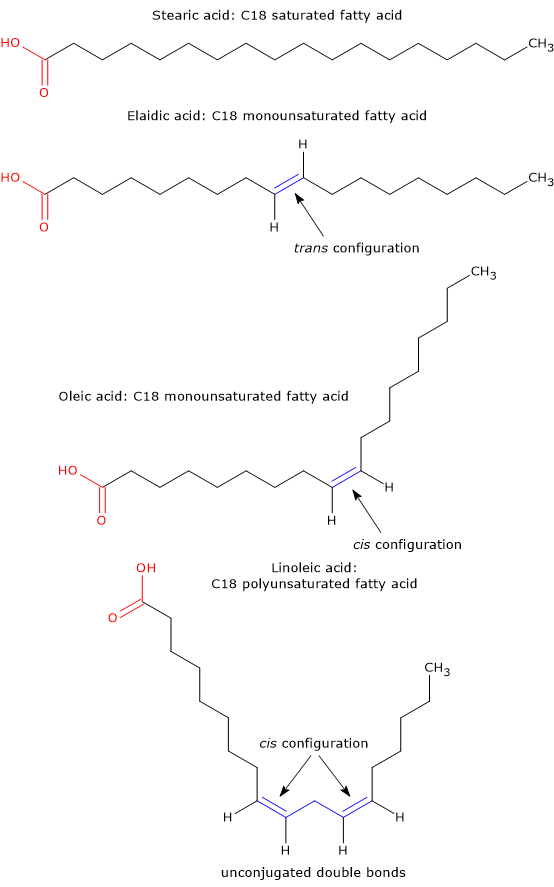 The double bond has a rigid planar structure and does not allow rotations between the two carbon atoms, which are possible in the single bond, and can be considered as a plane to which carbon chain attaches and continues, or enters and exits. If a part of the molecule has a rigid planar structure, the molecule can show geometric isomerism or cis–trans isomerism.
The double bond has a rigid planar structure and does not allow rotations between the two carbon atoms, which are possible in the single bond, and can be considered as a plane to which carbon chain attaches and continues, or enters and exits. If a part of the molecule has a rigid planar structure, the molecule can show geometric isomerism or cis–trans isomerism.
If the entry and exit of the chain from the plain occur on the same side, the double bond is in cis configuration, whereas if the entry and exit occur on opposite sides of the plain, the double bond is in trans configuration.
Cis configuration causes a bend or “kink” in the carbon chain, as a result of which chains pack less efficiently than those of saturated FAs, therefore establishing less intermolecular hydrophobic interactions. The resulting structure is less stable and less energy is needed to move the molecules away from each other. Hence, both the melting and boiling points of cis fatty acids will be lower than that of the corresponding saturated FAs.
Trans configuration has a geometry similar to that of the single bond, and straightens the carbon chain giving it a shape similar to that of a saturated FAs. Hence, molecules that have only trans double bonds pack with a similar efficiency to that of saturated FA, with which they have similar melting and boiling points.
The differences in the geometry between cis and trans double bonds and between saturated and unsaturated FAs affect their biological functions; for example, saturated and trans fatty acids form more rigid structures than cis fatty acids, and this affect the fluidity of biological membranes in which they are present.
Classification
There are several ways to classify fatty acids.
From a chemical point of view, they can be classified based on the characteristics of the carbon chains, such as the even or odd number of carbon atoms, the presence or absence of branches or cyclic structures, its length, the presence or absence of double/triple bonds, the number of double/triple bonds, the position of the first double bond with respect to the methyl end, the geometric isomerism of the double bonds, or whether they are present in free or esterified form.
Physiologically, they can be classified based on their essentiality for humans.
Length of the carbon chain
In fatty acids with linear chain, chain length varies from one up to 30 and more carbon atoms.
Based on the length of the chain, they can be classified into:
- short-chain fatty acids, in which chain length ranges from 1 to 5 carbons;
- medium-chain fatty acids, in which chain length ranges from 6 to 12 carbons;
- long-chain fatty acids, in which chain length ranges from 13 to 21 carbons;
- very long chain fatty acids, in which chain length is equal or greater than 22 carbons.
Saturated and unsaturated fatty acids
Based on the presence or absence of double/triple bonds in the carbon chain, they can be classified into:
- saturated FAs, when there is no double/triple bond;
- unsaturated FAs, when there is at least one double/triple bond.
Based on the number of double/triple bonds in the carbon chain, they can be classified into:
- monounsaturated FAs, when there is only one double/triple bond;
- polyunsaturated FAs (PUFAs), when there is more than one double/triple bond.
Unsaturated fatty acids can be further classified based on the position of the first double bond with respect to the terminal methyl end of the chain.
- Omega-3 polyunsaturated fatty acids, in which the first double bond is three carbon atoms from the methyl end, as in alpha-linolenic acid, stearidonic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA).
- Omega-6 polyunsaturated fatty acids, in which the first double bond is six carbon atoms from the methyl end, as in linoleic acid, gamma-linolenic acid, dihomo-gamma-linolenic acid, arachidonic acid (ARA), and adrenic acid.
- Omega-7 fatty acids, when the first double bond is seven carbon atoms from the methyl end, as in palmitoleic acid.
- Omega-9 fatty acids, when the first double bond is nine carbon atoms from the methyl end, as in oleic acid, erucic acid, nervonic acid and Mead acid.
- Omega-11 FA, when the first double bond is eleven carbon atoms from the methyl end, as in gadoleic acid.
Fatty acid is called acetylenic fatty acid when there is at least one triple bond in the carbon chain.
PUFAs can be classified also based on the presence of conjugated double bond systems.
- Conjugated FAs, when two or more double bonds are not separated by one or more methylene groups (–CH2–).
- Unconjugated FAs, when the double bonds in the chain are methylene-interrupted. The major polyunsaturated fatty acids are unconjugated fatty acids.
Cis and trans isomers
Unsaturated fatty acids containing double bonds can be classified based on the geometric isomerism into:
- cis fatty acids, more common in nature and in food;
- trans fatty acids or simply trans fats, less common.
Oleic acid and elaidinic or elaidic acid are examples of cis–trans isomerism: elaidic acid is the trans-isomer of oleic acid. Other trans fats found in food are vaccenic acid and brassidic acid.
Cyclic fatty acids
They contain a cyclic unit with three, five, like prostaglandins, or even six carbon atoms.
Non-esterified fatty acids
In nature, FAs are rarely found in free form, and in that case they are known as non-esterified fatty acids (NEFAs) or free fatty acids (FFAs). In humans, there is a small amount of NEFAs in the bloodstream, resulting from lipolysis, about three quarters of which are bound to albumin, whereas one quarter is bound to lipoproteins.
Most commonly, fatty acids are bound through ester bonds to other organic molecules such as glycerol, glycerol 3-phosphate and sterols, to form more complex lipids, such as triglycerides, phospholipids, and sterol esters, such as cholesterol esters, respectively.
Essential fatty acids
Fatty acids can be classified on a physiological basis, based on body’s ability to synthesize them. Alpha-linolenic acid and linoleic acid are classified as essential fatty acids as animals cannot synthesize them, lacking two desaturases: delta-12 desaturase (EC 1.14.19.6) and delta-15 desaturase (EC 1.14.19.13). Hence, they must be taken with food.
Functions
FAs have many roles in the cell.
- They are an energy source.
After being released from intracellular triglycerides, they are oxidized to produce ATP within the cell itself or, if released from adipose tissue triglycerides, in the cells of other tissues and organs. Liver, hearth and skeletal muscle are the main sites of oxidation.
Cells obtain more energy from their oxidation than from the oxidation of carbohydrates and proteins, on average 9 kcal/g against 4 kcal/g of proteins and carbohydrates, although the oxidation of short-chain saturated FAs yields less energy: for example, acetic acid, 3.5 kcal/g, propionic acid, 5.0 kcal/g of, butyric acid, 6.0 kcal/g, caproic acid, 7.5 kcal/g. - They are the precursors of potent bioactive lipid mediators that act as part of signal transduction pathways. Indeed, unsaturated fatty acids such as arachidonic acid and docosahexaenoic acid, deriving from blood stream or released from membrane phospholipids, can be metabolized to potent pro-resolving lipid mediators, such as Lipoxins, Maresins, D-series Resolvins, and to pro-inflammatory lipid mediators, such as Prostaglandins and Leukotrienes.
- They have a structural role in the formation of cell membranes, being a component of phospholipids which are key component of all biological membranes. And, unlike proteins and nucleic acids, have the ability to be incorporated into tissues intact, thereby altering tissue acyl compositions.
References
- Akoh C.C. and Min D.B. Food lipids: chemistry, nutrition, and biotechnology. 3th Edition. CRC Press, Taylor & Francis Group, 2008
- Burr G. and Burr M. A new deficiency disease produced by the rigid exclusion of fat from the diet. J Biol Chem 1929;82:345-367. doi:10.1016/S0021-9258(20)78281-5
- Calder P.C. Very long-chain n-3 fatty acids and human health: fact, fiction and the future. Proc Nutr Soc 2018;77(1):52-72. doi:10.1017/S0029665117003950
- Chow Ching K. Fatty acids in foods and their health implication. 3th Edition. CRC Press, Taylor & Francis Group, 2008
- Evans H. M. and G. O. Burr. A new dietary deficiency with highly purified diets. III. The beneficial effect of fat in the diet. Proc Soc Exp Biol Med 1928;25:390-397. doi:10.3181/00379727-25-3867
- Mozaffarian D., Katan M.B., Ascherio A., Stampfer M.J., Willett W.C. Trans fatty acids and cardiovascular disease. N Engl J Med 2006;354:1601-1613. doi:10.1056/NEJMra054035
- Petty H.R. Molecular biology of membranes: structure and function. Plenum Press, New York, 1993