Amylopectin is a highly branched polysaccharide made up of alpha-D-glucose units. Together with amylose, it is one of the two main constituents of starch granules, the means by which plants store energy and the most widespread and abundant form of carbohydrate storage on Earth.[12]
Glucose monomers are linked by α-(1→4) glycosidic bonds to form chains to which the branches are linked by α-(1→6) glycosidic bonds.[2]
Its synthesis requires the coordinated action of at least four distinct classes of enzymes: starch synthases (EC 2.4.1.21), starch branching enzymes (EC 2.4.1.18), starch debranching enzymes, and starch phosphorylase (EC 2.4.1.1).[17]
In starch granules, amylopectin is present in greater amount than amylose, and forms a semi-crystalline matrix within which amylose seems to be stored.
The amylose/amylopectin ratio significantly influences the physicochemical properties of starch, and consequently both its industrial applications, such as the production of food additives, and the possible health effects.[3]
Contents
- Structure of amylopectin
- Amylopectin synthesis
- Starch synthase
- Starch branching enzymes
- Starch debranching enzymes
- Starch phosphorylase
- Phosphorylation of amylopectin
- Amylose/amylopectin ratio
- References
Structure of amylopectin
Amylopectin is a highly branched polysaccharide, and has a molecular weight in the order of 107-108 Daltons, therefore much larger than amylose. It is formed of 104-105 glucose molecules which are linked by α-1,4 glycosidic bonds to form many relatively short chains, whose degree of polymerization is of 18-25 molecules.[17] The length of the chains vary depending on the source of the starch as well as the environmental and nutrient conditions during plant growth and seed formation.
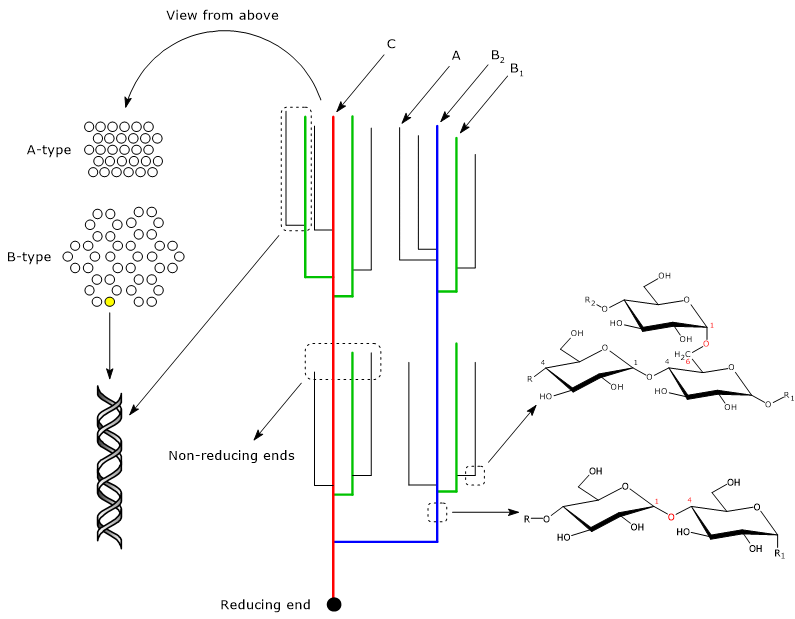
The chains are interconnected by α-1,6 glycosidic bonds to form a tree-like architecture, with neighboring chains forming cluster-like structures.[1]
In most starches, α-1,6 glycosidic bonds account for about 5 percent of all glycosidic bonds, a lower percentage than that found in glycogen molecule, about 9 percent, where the branches are more evenly distributed. The length and distribution of the branches directly affect the physicochemical properties of amylopectin, such as solubility, viscosity, ease of retrogradation, and gelatinization and pasting temperature.[3] For example, glycogen is water soluble whereas amylopectin and starch are not.
Amylopectin chains
Amylopectin chains can be classified on the basis of their length or the presence or absence of branches.
The classification according to the length identifies two main types of chains: short and long chains. Short chains have a degree of polymerization of 6-36 glucosyl units, although the upper limit depends on the source of amylopectin, whereas long chains have a degree of polymerization greater than or equal to 36. In most starches, the molar distribution of long to short chains is about 19-6, and is generally higher in A-crystalline starches, such as cereal endosperm starches, than in B-crystalline starches, such as those in potatoes.[17]
The classification on the basis of their connections to other chains identifies three categories: A-chains, B-chains and C-chains.[7]
- A-chains carry no branches, are short chains, with a degree of polymerization of about 13, and are the external chains.
- B-chains contain at least one branch, namely, A- and/or B-chain, are longer than A-chains, and are present in the inner part of the molecule. B-chains are in turn divided into B1-chains, with a polymerization degree of about 22, B2-chains, with a polymerization degree of about 42, B3-chains, with a polymerization degree of about 69, B4 and so on.
- C-chain is the B-chain which carries the sole reducing end.
A-chains and B1-chains participate in the formation of the clusters, while B2-, B3-, and B4-chains are thought to extend through two, three, and four clusters, respectively.[7][11]
A-type, B-type and C-type polymorphs
Within the clusters, neighboring linear chain segments form double helices, which run parallel to each other, with a period of 2.1 nm, and where each turn has six glucose molecules per chain.[10] The double helices form two types of crystalline structures called A-type polymorph, more dense and typical of cereal grains, and B-type polymorph, with a hexagonal structure, less dense, more hydrated, and typical of tuber and high amylose starches. Finally, C-type polymorph is a mixture of A-type and B-type polymorphs, and is found in the starches of root, legume and some fruits.[2]
This organization underlies the semi-crystalline nature of starch granules.
Growth rings
Although starch granules have different shapes, their internal architecture is remarkably conserved between different species. In fact, when viewed under a microscope, most starches exhibit a regular pattern of light and dark rings known as growth rings, so called because they resemble the growth rings of trees.[10]
The growth rings surround the hilum of the granule, namely, the core of the granule, whose exact structure is not known, although it appears to be formed by a relatively disordered alpha-glucan structure. The rings have a thickness of 200-400 nm, and are the result of alternating amorphous regions, less dense, and semi-crystalline regions, more dense.[10]
According to the cluster model, the semi-crystalline regions are due to the alternation of crystalline and amorphous lamellae, stacked with a periodicity of about 9-10 nm.[11] The crystalline lamellae are made up by the linear chains of amylopectin arranged to form the A-type, B-type or C-type polymorphs, and extend for about 6-7 nm, while the amorphous lamellae contain most of the branch points and extend for about 3nm.[5]
Amylopectin synthesis
The synthesis of amylopectin is believed to start from the hilum.[22]
The synthesis requires the coordinated activities of at least four distinct groups of enzymes: starch synthases, starch phosphorylase, starch branching enzymes and starch debranching enzymes.[12] Each group consists of several isoforms with distinct biochemical properties.
Like amylose synthesis, amylopectin synthesis requires short malto-oligosaccharides or MOS, α-(1→4)-glucans with a degree of polymerization of 2 to 7, which, acting as primers, are elongated by starch synthase, similarly to what happen with glycogenin in the initial steps of glycogen synthesis.[16]
MOS seem to have different origins, all of which require the activity of some enzymes involved in the synthesis of starch granules, namely:
- starch synthase III and starch phosphorylase, the latter in combination with the disproportionating enzyme (EC 2.4.1.25), which use ADP-glucose and glucose-1-phosphate as substrates, respectively;
- starch debranching enzymes, during the trimming of other amylopectin molecules.[16]
As MOS are poor solubility in aqueous environments, they seem to be able to evade the hydrolytic action of alpha-amylase (EC 3.2.1.1) and beta-amylase (EC 3.2.1.2).
The coordination, both spatial and temporal, of the involved enzymes, which in many cases also physically interact to form multienzyme complexes, is essential to allow the conversion of photosynthetic reaction products into the organized and insoluble structure of the polysaccharide. And, as in the case of amylose and glycogen synthesis, the polymerization of glucose into amylopectin, and more generally of osmotically active monosaccharides into osmotically inactive polysaccharides allows the storage of large amounts of monosaccharides inside the cell without any substantial increase in osmotic pressure.
Starch synthase
Six isoforms of starch synthase are known, all structurally related, of which five are involved in amylopectin synthesis, isoforms referred to as starch synthase I, II, III, IV and V or SSI, SSII, SSIII, SSIV and SSV, respectively, present in the stroma of plastids or portioned between the stroma and starch granules, whereas the sixth, the granule-bound starch synthase or GBSS (EC 2.4.1.242), is almost exclusively bound to granules and is involved in the synthesis of amylose.[10]
The first four isoforms have catalytic activity and belong, like GBSS, glycogen phosphorylase (EC 2.4.1.1) and glycogen synthase (EC 2.4.1.11), enzymes involved in glycogenolysis and glycogen synthesis, respectively, to the family of glycosyltransferases (EC 2.4). Conversely, starch synthase V has no catalytic activity.
During amylopectin synthesis, starch synthase catalyzes the adding of one glucose residue to the non-reducing end of a pre-existing α-(1→4) linked glucan chain. The monosaccharide is linked by a α-(1→4) glycosidic bond.[5]
[(1→4)-alpha-D-glucosyl](n) + ADP-alpha-D-glucose ⇌ [(1→4)-alpha-D-glucosyl](n+1) + ADP + H+
Note that, unlike glycogen synthase, starch synthase uses ADP-glucose and not UDP-glucose as the glucosyl donor.
The mode of action of starch synthase I, II, III, and IV is different from that of GBSS in that they are able to catalyze the addition of only one glucose unit per substrate encounter, a mode of action defined as distributive, whereas GBSS is able to catalyze the addition of more than one glucose unit per substrate encounter, a mode of action defined as processive.[17]
Roles of starch synthases
The initial steps of amylopectin synthesis, as well as the formation of a normal starch granule, require the presence of SSIV, although SSIII also appears to play a role, overlapping its action with that of SSIV.[16]
Like GBSS, SSIV requires the presence of a protein of the PTST family, PTST2, which has no catalytic activity, but is able, thanks to the presence of a specific domain able to bind carbohydrates, to facilitate the binding of the enzyme to alpha-glucans. SSIV is also able to dimerize, an important feature both for the catalytic activity and the ability to interact with other proteins.
According to a model of PTST2 action, the protein, by means of the domain able to bind to carbohydrates, recognizes and forms a complex with MOS having a specific three-dimensional helical shape. In turn, the protein-MOS complex interacts with a SSIV dimer, which is now able to catalyze the elongation of the alpha-glucan, while PTST2 is released so as to allow it to bind another malto-oligosaccharide and facilitate its subsequent interaction with another SSIV dimer.[15]
The action of SSIV is followed by that of the other isoforms of starch synthase. SSI catalyzes the elongation of malto-oligosaccharides with a degree of polymerization of 6 to 7, to form oligosaccharides with a degree of polymerization of 8 to 12, which, in turn, are excellent substrates for SSII, which catalyzes the synthesizes of chain with a degree of polymerization of 12 to 30. These alpha-glucans are further elongated by SSIII, to give linear chains with a degree of polymerization greater than 30.[12] Thus, SSIII appears to act not only in the initial steps of starch granule synthesis, but also in the later steps.
SSIV and SSV appear to be necessary for the synthesis of a regular number of starch granules of normal morphology.[1][16]
Starch branching enzymes
Starch branching enzymes catalyze the formation of α-(1→6) glycosidic bonds, therefore creating branch points in the linear chain of alpha-glucans, of which the main ones are glycogen and amylopectin.[20] Their action increase the number of non-reducing ends, which act as acceptors of glucose units in the elongation reactions.[14]
SBEs catalyze the hydrolytic cleavage of an α-(1→4) bond within an alpha-glucan chain, releasing an oligosaccharide whose reducing end is then linked to the hydroxyl group at C6 position of a glucosyl unit of a alpha-glucan chain. Therefore, the two chains are linked by α-(1→6)-glycosidic bond.
The chain to which the oligosaccharide is linked can be the same one from which it was detached, and in this case we speak of intra-chain transfer, or a different chain, and in this case we speak of inter-chain transfer.
Among the factors determining the type of transfer there seems to be the relative concentration of the linear α-(1→4) chains. In particular, it appears that closely associated chains, such as in the double helices in the clusters, promote inter-chain transfer.[19] Finally, it seems that the interaction between starch synthase I and starch branching enzyme is crucial in determining the bimodal chain length distribution observed in plant starches.
Starch branching enzyme isoforms
Two isoforms of the starch branching enzyme are present in plants, referred to as SBEI and SBEII.
Encoded by different genes, they have distinct biochemical properties, which suggests that they play different roles in determining the structure of amylopectin and amylose.[17]
SBEI appears to be expressed more in storage tissues, suggesting a significant role in determining the structural properties of storage starches, shows a substrate preference for amylose, and is able to transfer oligosaccharides with a degree of polymerization greater than 30, although most are between 10 and 13.[6] It also appears to be involved in the synthesis of super-long, or extra long, chains of amylopectin, whereas its other contributions to the structure of amylopectin appear to be less important. Not all plants express SBEI; for example, Arabidopsis and Canola (Brassica napus L.), which are oil-storing plants, have only SBEII and starch is present only in photosynthetic tissues.
SBEII is mostly expressed in grasses and cereals, and many other plants. Its loss causes important alterations in the architecture of amylopectin and reduce starch content. The enzyme shows a substrate preference for amylopectin and transfers oligosaccharides with a degree of polymerization of 6 to 14. In cereals and grasses, there are two tissue-specific isoforms encoded by distinct genes, termed SBEIIa, mainly present in the leaves, and SBEIIb, mainly present in the endosperm.[17]
Starch debranching enzymes
Starch debranching enzymes catalyze the hydrolysis of α-(1→6) glycosidic bonds, and are member of the alpha-amylase superfamily.
Two types of debranching enzymes are present in plants: isoamylases (EC 3.2.1.68) and pullulanases (EC 3.2.1.41).[8] Isoamylases acts on amylopectin and other polyglucans, whereas pullulanases debranch amylopectin and pullulan, a fungal polysaccharide. Isoamylase and pullulanase differ in substrate specificity as well, as they act on branches composed of at least three and two glycosidic residues, respectively.[18]
During starch granule formation, starch debranching enzymes play a crucial role in determining the water-insoluble properties and fine structure of amylopectin. Indeed, the enzyme activity is thought to allow the clustering of remaining branches, thus promoting interactions between adjacent chains and alpha-helix formation, which in turn appears to be important for the formation of the semi-crystalline structures of amylopectin, and then of starch.[17] In the semi-crystalline structure, the branches are presumably inaccessible to the action of starch debranching enzymes, alpha-amylase and beta-amylase.
Starch debranching enzymes are used industrially in the production of resistant starch and cyclodextrins, which are cyclic oligosaccharides.
Starch phosphorylase
The enzyme belongs, like starch synthases, to the family of glycosyltransferases, and is a phosphorylase which resembles glycogen phosphorylase. In plants, it is present in at least two isoenzymatic forms, Pho1, which is found in the stroma of plastids, and is thought to be the true starch phosphorylase involved in starch synthesis, and Pho2, isoform with cytosolic localization.[4]
Starch phosphorylase is thought to be involved in the initial steps of starch synthesis, catalyzing the reversible transfer of glucosyl units to an alpha-glucan, to which they are linked by a α-(1→4) glycosidic bond.[3] Unlike starch synthases, starch phosphorylase uses glucose-1-phosphate and not ADP-glucose as the glucosyl donor.[4]
Phosphorylation of amylopectin
Amylopectin, similarly to glycogen, binds phosphate groups in variable amounts depending on the botanical origin of the starch. For example, potato starch has a relatively high content of phosphate groups, with a degree of substitution of about 0.1 to 0.3 percent, whereas cereal endosperm starches have a phosphate content generally lower than 0.01 percent.[9]
The phosphorylations of amylopectin are catalyzed by two dikinases present in plastids: alpha-glucan water dikinase (EC 2.7.9.4) and phospho-glucan water dikinase (EC 2.7.9.5). These enzymes transfer the beta-phosphate group of ATP to a glucosyl unit of an alpha-glucan chain, while the gamma-phosphate group is transferred to water. Specifically, alpha-glucan water dikinase catalyzes the phosphorylation of the hydroxyl group at C6 position, whereas phospho-glucan water dikinase catalyzes the phosphorylation of the hydroxyl group at C3 position, generally of a prephosphorylated glucan chain.[13] About two thirds of the phosphate groups are bound at the C6 position, while about 20-30% at the C3 position. Phosphate groups are also present at the C2 position, although in a small percentage compared to the other positions. The enzyme which catalyze this phosphorylation in not known.
With regard to substrate specificity, it seems that phosphorylations accumulate more easily on longer chains. Furthermore, it seems to exist an inverse correlation between the total phosphate content and the frequency of amylopectin branching.
The negative charges carried by the phosphate groups cause the mutual repulsion between neighboring phosphorylated oligosaccharides. These repulsions appear to allow the opening and hydration of the chains, thus affecting the activity of the biosynthesis enzymes and making the chains more susceptible to attack by amylases as well.[21]
Amylose/amylopectin ratio
Starch granules are made up mostly of amylopectin and amylose.[12] The two polysaccharides are present in varying percentages, with amylose making up no more than 35 percent of the dry weight of the granule.[5] However, there are plants whose starch granules consist mostly, or almost exclusively, of amylopectin, and whose starches are defined as waxy starches, and plants whose starch granules consist mostly, or almost exclusively, of amylose.[19]
The amylose/amylopectin ratio influences the physicochemical properties of starch, such as the ability to absorb water, gelatinization, retrogradation, or resistance to enzymatic hydrolysis, the latter important in establishing the rate with which, during carbohydrate digestion, amylose and amylopectin are hydrolyzed to maltose and maltotriose by alpha-amylase.[3] Therefore, the amylose/amylopectin ratio influences the effects of the different types of starch on health, as well as their industrial uses.
References
- ^ a b Abt M.R., Pfister B., Sharma M., Eicke S., Bürgy L., Neale I., Seung D., Zeeman S.C. STARCH SYNTHASE5, a noncanonical starch synthase-like protein, promotes starch granule initiation in Arabidopsis. Plant Cell 2020;32(8):2543-2565. doi:10.1105/tpc.19.00946
- ^ a b Cornejo-Ramírez Y.I., Martínez-Cruz O., Del Toro-Sánchez C.L., Wong-Corral F.J., Borboa-Flores J. & Cinco-Moroyoqui F.J. The structural characteristics of starches and their functional properties. CYTA J Food 2018;16(1):1003-1017. doi:10.1080/19476337.2018.1518343
- ^ a b c d Crofts N., Abe N., Oitome N.F., Matsushima R., Hayashi M., Tetlow I.J., Emes M.J., Nakamura Y., Fujita N. Amylopectin biosynthetic enzymes from developing rice seed form enzymatically active protein complexes. J Exp Bot 2015;66(15):4469-82. doi:10.1093/jxb/erv212
- ^ a b Cuesta-Seijo J.A., Ruzanski C., Krucewicz K., Meier S., Hägglund P., Svensson B., Palcic M.M. Functional and structural characterization of plastidic starch phosphorylase during barley endosperm development. PLoS One 2017;12(4):e0175488. doi:10.1371/journal.pone
- ^ a b c Gous P.W., Fox G.P. Review: Amylopectin synthesis and hydrolysis – Understanding isoamylase and limit dextrinase and their impact on starch structure on barley (Hordeum vulgare) quality. Trends Food Sci Technol 2017;62:23-32. doi:10.1016/j.tifs.2016.11.013
- ^ Guan H.P., Preiss J. Differentiation of the properties of the branching isozymes from maize (Zea mays). Plant Physiol 1993;102(4):1269-1273. doi:10.1104/pp.102.4.1269
- ^ a b Li G., Yacine Y., Zhu F. Relationships between supramolecular organization and amylopectin fine structure of quinoa starch. Food Hydrocoll 2021;117:106685. doi:10.1016/j.foodhyd.2021.106685
- ^ Møller M.S., Henriksen A., Svensson B. Structure and function of α-glucan debranching enzymes. Cell Mol Life Sci. 2016;73(14):2619-41. doi:10.1007/s00018-016-2241-y
- ^ Nitschke F., Wang P., Schmieder P., Girard J.M., Awrey D.E., Wang T., Israelian J., Zhao X., Turnbull J., Heydenreich M., Kleinpeter E., Steup M., Minassian B.A. Hyperphosphorylation of glucosyl C6 carbons and altered structure of glycogen in the neurodegenerative epilepsy Lafora disease. Cell Metab 2013;17(5):756-67. doi:10.1016/j.cmet.2013.04.006
- ^ a b c d Pfister B., Zeeman S.C. Formation of starch in plant cells. Cell Mol Life Sci 2016;73(14):2781-807. doi:10.1007/s00018-016-2250-x
- ^ a b Pfister B., Zeeman S.C., Rugen M.D., Field R.A., Ebenhöh O., Raguin A. Theoretical and experimental approaches to understand the biosynthesis of starch granules in a physiological context. Photosynth Res 2020;145:55-70. doi:10.1007/s11120-019-00704-y
- ^ a b c d Qu J., Xu S., Zhang Z., Chen G., Zhong Y., Liu L., Zhang R., Xue J., Guo D. Evolutionary, structural and expression analysis of core genes involved in starch synthesis. Sci Rep 2018;8(1):12736. doi:10.1038/s41598-018-30411-y
- ^ Ritte G., Heydenreich M., Mahlow S., Haebel S., Kötting O., Steup M. Phosphorylation of C6- and C3-positions of glucosyl residues in starch is catalysed by distinct dikinases. FEBS Lett 2006;580(20):4872-6. doi:10.1016/j.febslet.2006.07.085
- ^ Sawada T., Nakamura Y., Ohdan T., Saitoh A., Francisco P.B. Jr, Suzuki E., Fujita N., Shimonaga T., Fujiwara S., Tsuzuki M., Colleoni C., Ball S. Diversity of reaction characteristics of glucan branching enzymes and the fine structure of α-glucan from various sources. Arch Biochem Biophys 2014;562:9-21. doi:10.1016/j.abb.2014.07.032
- ^ Seung D., Boudet J., Monroe J., Schreier T.B., David L.C., Abt M., Lu K.J., Zanella M., Zeeman S.C. Homologs of PROTEIN TARGETING TO STARCH control starch granule initiation in Arabidopsis leaves. Plant Cell 2017;29(7):1657-1677. doi:10.1105/tpc.17.00222
- ^ a b c d Szydlowski N., Ragel P., Raynaud S., Lucas M.M., Roldán I., Montero M., Muñoz F.J., Ovecka M., Bahaji A., Planchot V., Pozueta-Romero J., D’Hulst C., Mérida A. Starch granule initiation in Arabidopsis requires the presence of either class IV or class III starch synthases. Plant Cell 2009;21(8):2443-57. doi:10.1105/tpc.109.066522
- ^ a b c d e f g Tetlow I.J., Bertoft E. A review of starch biosynthesis in relation to the building block-backbone model. Int J Mol Sci 2020;21(19):7011. doi:10.3390/ijms21197011
- ^ Xia W., Zhang K., Su L., Wu J. Microbial starch debranching enzymes: developments and applications. Biotechnol Adv 2021;50(3):107786. doi:10.1016/j.biotechadv.2021.107786
- ^ a b Wang J., Hu P., Lin L., Chen Z., Liu Q., Wei C. Gradually decreasing starch branching enzyme expression is responsible for the formation of heterogeneous starch granules. Plant Physiol 2018;176(1):582-595. doi:10.1104/pp.17.01013
- ^ Wilkens C., Svensson B., Møller M.S. Functional roles of starch binding domains and surface binding sites in enzymes involved in starch biosynthesis. Front Plant Sci 2018;9:1652. doi:10.3389/fpls.2018.01652
- ^ Zhou W., He S., Naconsie M., Ma Q., Zeeman S.C., Gruissem W. & Zhang P. Alpha-glucan, water dikinase 1 affects starch metabolism and storage root growth in Cassava (Manihot esculenta Crantz). Sci Rep 2017;7:9863 doi:10.1038/s41598-017-10594-6
- ^ Ziegler G.R., Creek J.A., Runt J. Spherulitic crystallization in starch as a model for starch granule initiation. Biomacromolecules 2005;6(3):1547-54. doi:10.1021/bm049214p