Starch synthase (EC 2.4.1.21) is an enzyme that catalyzes the transfer of glucose molecules from ADP-glucose to the non-reducing end of a pre-existing α-(1→4) linked glucan chain, to which the monosaccharides are linked by an α-(1→4) glycosidic bond.[4]
[(1→4)-alpha-D-glucosyl](n) + ADP-alpha-D-glucose ⇌ [(1→4)-alpha-D-glucosyl](n+1) + ADP + H+
This enzymatic activity is involved in the synthesis of amylose and amylopectin, the two major constituents of starch, which is the main storage form of carbohydrates in plants.[7]
Starch synthase belongs to the family of glycosyltransferases (EC 2.4), like starch phosphorylase (EC 2.4.1.1), another enzyme involved in starch metabolism, and glycogen synthase (EC 2.4.1.11) and glycogen phosphorylase (EC 2.4.1.1), enzymes involved in glycogen synthesis and glycogen degradation or glycogenolysis, respectively.[6] However, while glycogen synthase uses UDP-glucose as the glucosyl donor, and starch phosphorylase uses glucose 1-phosphate, starch synthase uses ADP-glucose.[2][3]
Contents
- Isoforms
- Starch synthase and MOS
- Starch synthase and amylopectin synthesis
- GBSS and amylose synthesis
- References
Isoforms
Six isoforms of starch synthase are known in plants. They are structurally related proteins, with five involved in the synthesis of amylopectin, referred to as starch synthase I, II, III, IV and V, or SSI, SSII, SSIII, SSIV and SSV, respectively, and one involved in the synthesis of amylose, the granule-bound starch synthase (GBSS) (EC 2.4.1.242).[6] GBSS, SSI, SSII, SSIII, and SSIV have catalytic activity, whereas SSV lacks catalytic activity.[1]
GBSS is almost exclusively bound to starch granules and is located mostly within them, as evidenced by treatment of the granule surface with proteases. The other isoforms of starch synthase are present either only in the stroma of the plastids or portioned between starch granules and the stroma, and are called soluble starch synthases.[8]
Starch synthases I, II, III, and IV catalyze the adding of one glucose residue per substrate encounter, a distributive mode of action, whereas GBSS catalyze the addition of more than one glucosidic unit per substrate encounter, a processive mode of action.[11]
Starch synthase and MOS
Starch synthases involved in the early steps of amylose and amylopectin synthesis require short malto-oligosaccharides (MOS) to initiate de novo synthesis of the two polysaccharides.[11]
These small oligosaccharides, namely, α-(1→4)-glucans with a degree of polymerization of 2 to 7, act as primers and are elongated, a function analogous to that performed by glycogenin in glycogen synthesis.
MOS can originate from the activity of starch synthase III, starch phosphorylase, or starch debranching enzymes.
Being poorly water soluble, MOS seem able to evade the hydrolytic action of alpha-amylase (EC 3.2.1.1) and beta-amylase.[8]
Starch synthase and amylopectin synthesis
The synthesis of amylopectin requires the temporally coordinated action of at least four classes of enzymes, namely, starch synthase isoenzymes, starch phosphorylase, starch branching enzymes (EC 2.4.1.18), and starch debranching enzymes.[2][7] It is also believed that, in many cases, these enzymes physically interact with each other to form multienzyme complexes, which are structures capable of increasing the efficiency of a metabolic pathway.[11]
It is generally accepted that the growth of the starch granule occurs from a central core called the hilum, whose precise structure is unknown, although it appears to be formed by a disordered structure of α-glucans.[13] The initiation of the hilum, the formation of a normal starch granule morphology, and the degree of starch accumulation require the presence of SSIV, although it has been suggested that SSIII and SSV may also play a role, overlapping their activity with that of SSIV.[10]
GBSS and amylose synthesis
Granule-bound starch synthase is involved in the synthesis of amylose.
This enzyme was first reported by Luis Federico Leloir in the early 60s, the same researcher who in 1948 had discovered the main pathway for galactose metabolism, the Leloir pathway.[5]
Its catalytic action is not entirely simultaneous with that of the other starch synthases, as it requires the presence of an amylopectin-matrix.[6]
In grasses, the granule-bound starch synthase is present in two isoforms, encoded by distinct genes, and referred to as GBSSI and GBSSII.[12]
GBSS requires, for its catalytic activity, the presence of a protein of the PTST family, PTST1, which allows its binding to the starch granule, and whose action seems to be more important in chloroplasts than in amyloplasts.[9]
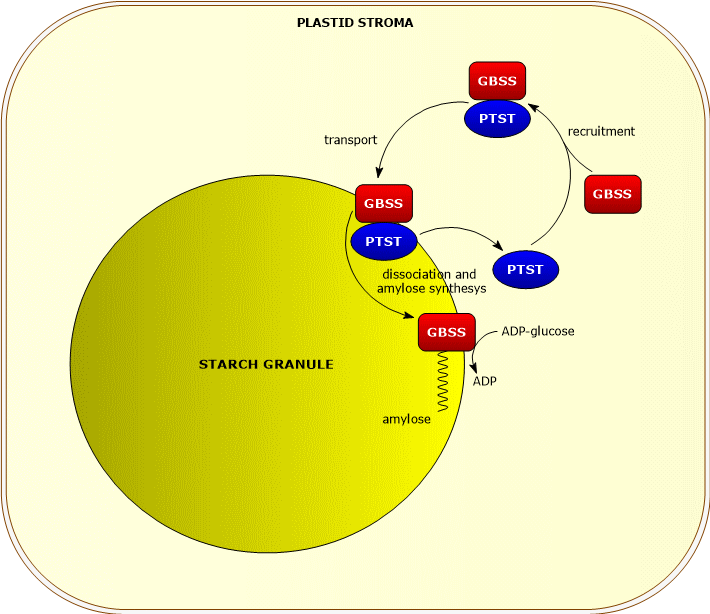 PTST1 appears to associate, in the plastid stoma, with GBSS. The complex binds to the nascent starch granule, the protein dissociates from the enzyme, that begins to catalyze the elongation of the malto-oligosaccharides, while the protein returns to the stroma where it recruits another GBSS.
PTST1 appears to associate, in the plastid stoma, with GBSS. The complex binds to the nascent starch granule, the protein dissociates from the enzyme, that begins to catalyze the elongation of the malto-oligosaccharides, while the protein returns to the stroma where it recruits another GBSS.
References
- ^ Abt M.R., Pfister B., Sharma M., Eicke S., Bürgy L., Neale I., Seung D., Zeeman S.C. STARCH SYNTHASE5, a noncanonical starch synthase-like protein, promotes starch granule initiation in Arabidopsis. Plant Cell 2020;32(8):2543-2565. doi:10.1105/tpc.19.00946
- ^ a b Crofts N., Abe N., Oitome N.F., Matsushima R., Hayashi M., Tetlow I.J., Emes M.J., Nakamura Y., Fujita N. Amylopectin biosynthetic enzymes from developing rice seed form enzymatically active protein complexes. J Exp Bot 2015;66(15):4469-82. doi:10.1093/jxb/erv212
- ^ Cuesta-Seijo J.A., Ruzanski C., Krucewicz K., Meier S., Hägglund P., Svensson B., Palcic M.M. Functional and structural characterization of plastidic starch phosphorylase during barley endosperm development. PLoS One 2017;12(4):e0175488. doi:10.1371/journal.pone
- ^ Gous P.W., Fox G.P. Review: Amylopectin synthesis and hydrolysis – Understanding isoamylase and limit dextrinase and their impact on starch structure on barley (Hordeum vulgare) quality. Trends Food Sci Technol 2017;62:23-32. doi:10.1016/j.tifs.2016.11.013
- ^ Leloir L.F., de Fekete M.A., Cardini C.E. Starch and oligosaccharide synthesis from uridine diphosphate glucose. J Biol Chem 1961;236:636-41. doi:10.1016/S0021-9258(18)64280-2
- ^ a b c Pfister B., Zeeman S.C. Formation of starch in plant cells. Cell Mol Life Sci 2016;73(14):2781-807. doi: 10.1007/s00018-016-2250-x
- ^ a b Qu J., Xu S., Zhang Z., Chen G., Zhong Y., Liu L., Zhang R., Xue J., Guo D. Evolutionary, structural and expression analysis of core genes involved in starch synthesis. Sci Rep 2018;8(1):12736. doi:10.1038/s41598-018-30411-y
- ^ a b Seung D., Boudet J., Monroe J., Schreier T.B., David L.C., Abt M., Lu K.J., Zanella M., Zeeman S.C. Homologs of PROTEIN TARGETING TO STARCH control starch granule initiation in Arabidopsis leaves. Plant Cell 2017;29(7):1657-1677. doi:10.1105/tpc.17.00222
- ^ Seung D., Soyk S., Coiro M., Maier B.A., Eicke S., and Zeeman S.C. PROTEIN TARGETING TO STARCH is required for localising GRANULE-BOUND STARCH SYNTHASE to starch granules and for normal amylose synthesis in Arabidopsis. PLoS Biol 2015;13:e1002080. doi:10.1371/journal.pbio.1002080
- ^ Szydlowski N., Ragel P., Raynaud S., Lucas M.M., Roldán I., Montero M., Muñoz F.J., Ovecka M., Bahaji A., Planchot V., Pozueta-Romero J., D’Hulst C., Mérida A. Starch granule initiation in Arabidopsis requires the presence of either class IV or class III starch synthases. Plant Cell 2009;21(8):2443-57. doi:10.1105/tpc.109.066522
- ^ a b c Tetlow I.J., Bertoft E. A review of starch biosynthesis in relation to the building block-backbone model. Int J Mol Sci 2020;21(19):7011. doi:10.3390/ijms21197011
- ^ Vrinten P.L., Nakamura T. Wheat granule-bound starch synthase I and II are encoded by separate genes that are expressed in different tissues. Plant Physiol 2000;122(1):255-64. doi:10.1104/pp.122.1.255
- ^ Ziegler G.R., Creek J.A., Runt J. Spherulitic crystallization in starch as a model for starch granule initiation. Biomacromolecules 2005;6(3):1547-54. doi:10.1021/bm049214p