Different methods of protein classification have been proposed, but currently none of them is universally valid.
Below, some examples based on chemical composition, structure, functions, and solubility in different solvents.
Contents
- Protein classification based on chemical composition
- Protein classification based on shape
- Protein classification based on biological functions
- Classification based on solubility
- References
Protein classification based on chemical composition
On the basis of their chemical composition, proteins may be divided into two classes: simple and complex.
Simple proteins
Also known as homoproteins, they are made up of only amino acids. Examples are plasma albumin, collagen, and keratin.
Conjugated proteins
Sometimes also called heteroproteins, they contain in their structure a non-protein portion.
Three examples are glycoproteins, chromoproteins, and phosphoproteins.
- Glycoproteins
They are proteins that covalently bind one or more carbohydrate units to the polypeptide backbone.
Typically, the branches consist of not more than 15-20 carbohydrate units, where you can find arabinose, fucose (6-deoxygalactose), galactose, glucose, mannose, N-acetylglucosamine (GlcNAc, or NAG), and N-acetylneuraminic acid (Neu5Ac or NANA).
Examples of glycoproteins are:
glycophorin, the best known among erythrocyte membrane glycoproteins;
fibronectin, that anchors cells to the extracellular matrix through interactions on one side with collagen or other fibrous proteins, while on the other side with cell membranes;
all blood plasma proteins, except albumin;
immunoglobulins or antibodies.
- Chromoproteins
They are proteins that contain colored prosthetic groups.
Typical examples are:
hemoglobin and myoglobin, which bind, respectively, one and four heme groups;
chlorophylls, which bind a porphyrin ring with a magnesium atom at its centre;
rhodopsins, which bind retinal.
- Phosphoproteins
They are proteins that bind phosphoric acid to serine and threonine residues.
Generally, they have a structural function, such as tooth dentin, or reserve function, such as milk caseins (alpha, beta, gamma and delta), and egg yolk phosvitin.
Protein classification based on shape
On the basis of their shape, proteins may be divided into two classes: fibrous and globular.
Fibrous proteins
They have primarily mechanical and structural functions, providing support to the cells as well as the whole organism.
These proteins are insoluble in water as they contain, both internally and on their surface, many hydrophobic amino acids. The presence on their surface of hydrophobic amino acids facilitates their packaging into very complex supramolecular structures.
Regarding this, it should be noted that their polypeptide chains form long filaments or sheets, where, in most cases, only one type of repeating secondary structure is found..
Among vertebrates, these proteins provide external protection, support, and shape. Thanks to their structural properties, they ensure flexibility and/or strength.
Some fibrous proteins, such as alpha-keratins, are only partially hydrolyzed in the intestine.
Here are some examples.
- Fibroin
It is produced by spiders and insects. An example is that produced by the silkworm, Bombyx mori. - Collagen
The term “collagen” indicates not a single protein but a family of structurally related proteins (at least 29 different types), which constitute the main protein component of connective tissue, and more generally, the extracellular scaffolding of multicellular organisms. In vertebrates, they represent about 25-30 percent of all proteins.
They are found in different tissues and organs, such as tendons and the organic matrix of bone, where they are present in very high percentages, but also in cartilage and in the cornea of the eye.
In the different tissues, they form different structures, each capable of satisfying a particular need. For example, in the cornea, the molecules are arranged in an almost crystalline array, so that they are virtually transparent, while in the skin they form fibers not very intertwined and directed in all directions, which ensure the tensile strength of the skin itself. - alpha-Keratins
They constitute almost the entire dry weight of nails, claws, beak, hooves, horns, hair, wool, and a large part of the outer layer of the skin.
The different stiffness and flexibility of these structures is a consequence of the number of disulfide bonds that contribute, together with other binding forces, to stabilize the protein structure. And this is the reason why wool keratins, which have a low number of disulfide bonds, are flexible, soft and extensible, unlike claw and beak keratins that are rich in disulfide bonds. - Elastin
This protein provides elasticity to the skin and blood vessels, a consequence of its random coiled structure, that differs from the structures of the α-keratins and collagens.
Note: the different types of collagen have low nutritional value as deficient in several amino acids. In fact, they contain no tryptophan and low amount of the other essential amino acids.
The gelatin used in food preparation is a derivative of collagen.
Globular proteins
Most of the proteins belong to this class.
They have a compact and more or less spherical structure, more complex than fibrous proteins. In this regard, motifs, domains, tertiary and quaternary structures are found, in addition to the secondary structures.
They are generally soluble in water but can also be embedded in biological membranes as transmembrane proteins, thus being in a hydrophobic environment.
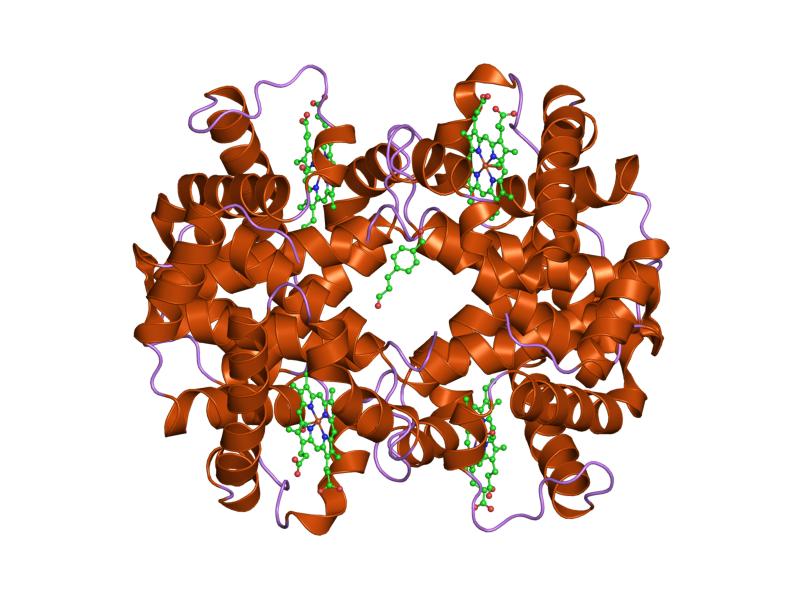
Unlike fibrous proteins, that have structural and mechanical functions, they act as:
- enzymes;
- hormones;
- membrane transporters and receptors;
- transporters of triglycerides and fatty acids, two classes of lipids, and oxygen in the blood;
- immunoglobulins or antibodies;
- grain and legume storage proteins.
Examples of globular proteins are myoglobin, hemoglobin, and cytochrome c.
At the intestinal level, most of the globular proteins of animal origin are hydrolyzed almost entirely to amino acids.
Protein classification based on biological functions
The multitude of functions that proteins perform is the consequence of both the folding of the polypeptide chain, therefore of their three-dimensional structure, and the presence of many different functional groups in the amino acid side chains, such as thiols, alcohols, thioethers, carboxamides, carboxylic acids and different basic groups.
From the functional point of view, they may be divided into several groups.
- Enzymes.
In living organisms, almost all reactions are catalyzed by specific proteins called enzymes. They have a high catalytic power, increasing the rate of the reaction in which they are involved at least by factor 106. Therefore, life as we know could not exist without their “facilitating action”.
Almost all known enzymes, and in the human body they are thousand, are proteins, except some catalytic RNA molecules called ribozymes, that is, ribonucleic acid enzymes. - Transport proteins
Many small molecules, organic and inorganic, are transported in the bloodstream and extracellular fluids, across the cell membranes, and inside the cells from one compartment to another, by specific proteins.
Examples are:
hemoglobin, that carries oxygen from the alveolar blood vessels to tissue capillaries;
transferrin, which carries iron in the blood;
membrane carriers;
fatty acid binding proteins (FABP), that is, the proteins involved in the intracellular transport of fatty acids;
proteins of plasma lipoproteins, macromolecular complexes of proteins and lipids responsible for the transport of triglycerides, which are otherwise insoluble in water;
albumin, that carries free fatty acids, bilirubin, thyroid hormones, and certain medications such as aspirin and penicillin, in the blood.
Many of these proteins also play a protective role, since the bound molecules, such as fatty acids, may be harmful for the organism when present in free form.
- Storage proteins
Examples are:
ferritin, that stores iron intracellularly in a non-toxic form;
milk caseins, that act as a reserve of amino acids for the milk;
egg yolk phosvitin, that contains high amounts of phosphorus;
prolamins and glutelins, the storage proteins of cereals.
- Mechanical support
Proteins have a pivotal role in the stabilization of many structures. Examples are alpha-keratins, collagen and elastin. The same cytoskeletal system, the scaffold of the cell, is made of proteins. - They generate movement.
They are responsible, among others, for:
contraction of the muscle fibers, of which myosin is the main component;
the propulsion of spermatozoa and microorganisms with flagella;
the separation of chromosomes during mitosis.
- They are involved in nerve transmission.
An example is the receptor for acetylcholine at synapses. - They control development and differentiation.
Some proteins are involved in the regulation of gene expression. An example is the nerve growth factor (NGF), discovered by Rita Levi-Montalcini, that plays a leading role in the formation of neural networks. - Hormones
Many hormones are proteins.
They are regulatory molecules involved in the control of many cellular functions, from metabolism to reproduction. Examples are insulin, glucagon, and thyroid-stimulating hormone (TSH). - Protection against harmful agents.
The antibodies or immunoglobulins are glycoproteins that recognize antigens expressed on the surface of viruses, bacteria and other infectious agents.
Interferon, fibrinogen, and factors of blood coagulation are other members of this group. - Storage of energy.
Proteins, and in particular the amino acids that constitute them, act as energy storage, second in size only to the adipose tissue, that in particular conditions, such as prolonged fasting, may become essential for survival. However, their reduction of more than 30 percent leads to a decrease of the contraction capacity of respiratory muscle, immune function, and organ function, that are not compatible with life. Therefore, proteins are an extremely valuable fuel.
Classification based on solubility
The different globular proteins can be classified based on their solubility in different solvents, such as water, salt and alcohol (see: Gluten).
References
- Kessel A., Ben-Tal N. Introduction to proteins: structure, function, and motion. CRC Press, 2011. doi:10.1002/cbic.201100254
- Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 6th Edition. W.H. Freeman and Company, 2012
- Stipanuk M.H., Caudill M.A. Biochemical, physiological, and molecular aspects of human nutrition. 3rd Edition. Elsevier health sciences, 2012