Glycogen is a branched polymer made up of D-glucose units, and serves as storage form of the monosaccharide, and therefore of energy.
As it is synthesized without a template, unlike proteins and nucleic acids it exists as a population of molecules with different chemical structures and sizes.
Due to the branched structure, glycogen is a compact and soluble macromolecule, has a low osmotic pressure, and allows rapid release of the stored glucose when needed.
Glycogen molecule forms assemblages with proteins that are essential for its metabolism, such as glycogenin (EC 2.4.1.186), glycogen synthase (EC 2.4.1.11), glycogen phosphorylase (EC 2.4.1.1), debranching enzyme (EC 2.4.1.25 and EC 3.2.1.33), and others that regulate its anchoring to cytoskeleton and membranes. Such polysaccharide-protein aggregates are called beta granules and are found in the cytosol of bacteria, Archea, Fungi and animal cells. Furthermore, phosphate groups are covalently linked to the polysaccharide.
Proteins and phosphate groups seem to be involved in the regulation of its metabolism that, as well as metabolism in general, has two phase: catabolism, the destructive phase, and anabolism, the constructive phase. During the constructive phase, reactions catalyzed by specific enzymes lead to glycogen synthesis, whereas during the destructive phase, reactions catalyzed by specific enzymes lead to glycogen breakdown or glycogenolysis.
In animals, it is found in practically all cells and, in mammals, it is most abundant in the liver and skeletal muscle. Within the liver, several beta granules arrange to form the so-called alpha granules.Glycogen is also found in lysosomes.
In humans, it represents less than 1 percent of the body’s energy stores, and is essential in maintaining blood glucose homeostasis, too.
It is absent in plants, where starch is the storage form of glucose. Therefore, the polymerization of glucose represents a universal mechanism for energy storage.
From a human nutrition point of view, glycogen has little significance as after an animal has been killed it is mostly broken down to glucose and then to lactic acid. It should be noted that the acidity consequently to lactic acid production gradually improves the texture and keeping qualities of the meat. The only dietary sources are oysters and other shellfish that are eaten virtually alive; they contain about 5 percent glycogen.
Contents
- The discovery
- Chemical and molecular structure of glycogen
- Beta granules
- Alpha granules
- Glycogen metabolism: the constructive phase
- Glycogen metabolism: the degradative phase
- Coordinated regulation of glycogen metabolism
- Where is glycogen found in humans?
- Why is it important to humans?
- Glycogen and muscle work
- Energy yield under anaerobic conditions
- Energy yield under aerobic conditions
- References
The discovery
Glycogen was discovered in 1857 by the French physiologist Claude Bernard, considered the founder of experimental medicine. In the second half of the last century, studies on glycogen metabolism led to several significant discoveries, such as:
- the reversible phosphorylation of proteins;
- protein kinases and protein phosphatases;
- the effect of insulin on the activity of intracellular enzymes.
In turn, this led to the award of four Nobel Prizes, three for physiology or medicine, to Carl Ferdinand Cori and Gerty Theresa Cori, née Radnitz in 1947, Earl Sutherland Jr. in 1971, and Edwin Krebs and Edmond Fischer in 1992, and one for chemistry, to Louis Leloir in 1970.
Chemical and molecular structure of glycogen
Individual glycogen molecule is a branched polymer of D-glucose in the pyranose form, namely, a stiff six-membered heterocyclic ring, five carbons and one oxygen, with chair conformation.
The central priming protein glycogenin and phosphate groups are covalently bound to the polysaccharide chain.
Most of the glucose units are linked by α-1,4 glycosidic bonds, where each unit is linked to the next by a bond between C-1 of one unit and the hydroxyl group on the C-4 of the next unit, with an oxygen atom acting as a bridge between the two carbon atoms.
The branch points are introduced by α-1,6 glycosidic bonds, that occur approximately every 8-12 residues, again with an oxygen atom acting as a bridge between the two carbon atoms, in this case, C-1 and C-6, and with an average chain length of about 13 residues in mammals. Because each branch ends in a non-reducing residue, there are n+1 non-reducing ends in the molecule, where n is number of chains, but only one reducing end to which glycogenin is linked.
Note: in disaccharides, oligosaccharides, and polysaccharide the non-reducing end is the end that lacks a free anomeric carbon atom.
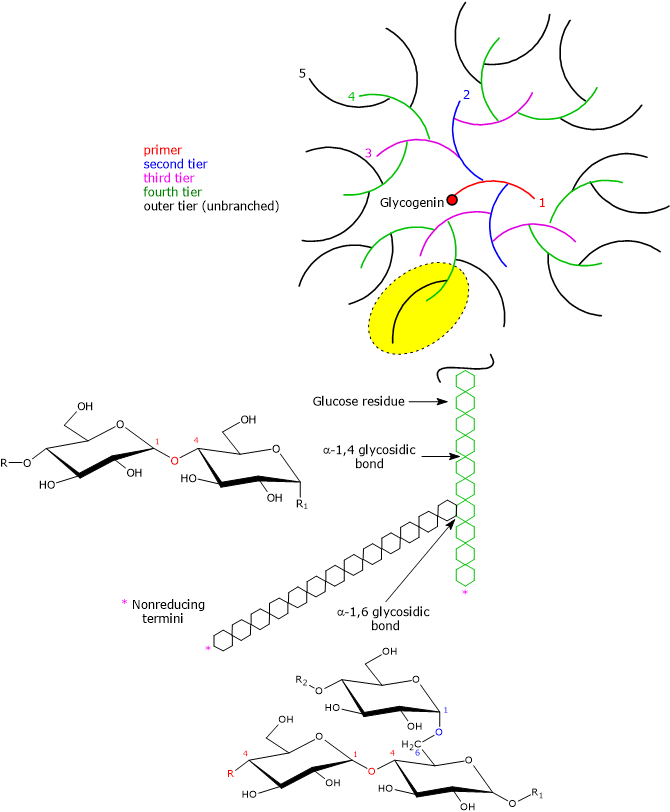
Having the same types of bonds, the primary structure of glycogen resembles that of amylopectin, which, with amylose, is one of the two polymers of D-glucose units composing starch. However, compared to amylopectin where branches occur every 25-30 glucose units, glycogen is more branched, and the branches are smaller.
Unlike proteins and nucleic acids, polysaccharides are synthesized without a template, resulting from the addition of monosaccharides or oligosaccharides to the growing structure. In addition, because branches occur without a precise localization, molecules with the same mass will not necessarily have the same structure. Hence, for each type of molecules there are different chemical structures.
Moreover, glycogen isolated from different biological sources exists as a population of molecules of different sizes. Therefore, the best way to describe its chemistry is to define the distribution of the molecular masses, and the average frequency with which branches occurs and their average length.
Finally, it should be emphasized that glycogen is not a static entity but constantly vary over the course of its existence.
As glucose has chirality centers, it exists as a pair of enantiomers, indicated according to the Fischer-Rosanoff convention as D-glucose, the most widespread in nature and the monomeric unit of glycogen and starch, and L-glucose.
Who does stabilize 3D structure?
The folding into three-dimensional structures of macromolecules such as proteins, nucleic acids and polysaccharides is governed by the same principles: the monomeric units, namely, amino acids, nucleotides, and monosaccharides, with their more-or-less rigid structure, are joined by covalent bonds to form one dimensional polymers that spontaneously fold into three-dimensional structures stabilized by noncovalent interactions such as:
- hydrogen bonds;
- van der Waals interactions;
- hydrophobic interactions;
- ionic interactions, when charged subunits are present.
These interactions can occur within macromolecules or between macromolecules, as in supramolecular complexes such as cellulose or multienzyme complexes.
As the pyranose ring of glucose is a rigid structure, the three-dimensional conformation of the oligosaccharides and polysaccharides results from rotation about both C−O bonds of the glycosidic bond, with the bond angles labeled Phi and Psi. However, there is no free rotation about each C−O bonds due to the steric interference by substituents. Hence, some conformations will be more stable than others. For amylose and glycogen, the most stable 3D structure is a tightly coiled helix stabilized by interchain hydrogen bonds.
What are the advantages of the branched structure?
The highly branched structure of glycogen offer several advantages.
- The non-reducing ends present on the outermost tier can act as a substrate for glycogen phosphorylase. Therefore, many glycogen phosphorylases can work simultaneously allowing a rapid mobilization of stored glucose as glucose 1-phosphate.
- The highly branched structure allows stored glucose to exert a much lower osmotic pressure than it would exert if it were in its monomeric form. For example, hepatocytes store an amount of glucose that, in free form, would have a concentration of about 0.4 M, against a glycogen concentration of about 0.01 mM. Therefore, if glucose were in free form, the resulting osmolarity would be so elevated to cause an osmotic entry of water that would lead to cell lysis. Moreover, as extracellular concentration of glucose is about 5 mM, glucose uptake into a cell with a glucose concentration of 0.4 M would be particularly energetically expensive.
- Branches allow the formation of compact granules.
- If branches were absent or at least few, a very large number of long linear polymers would have to be present to have a number of non-reducing ends comparable to those present in glycogen and to store a comparable amount of glucose. This could cause cell damage. Evidence in favor of this hypothesis comes from a rare genetic disease, Anderson’s disease or amylopectinosis or glycogen storage disease type 4, due to mutations in the gene for branching enzyme. These mutations lead to a deficiency of the enzyme activity and accumulation in different tissues of abnormally branched glycogen that resembles amylopectin.
- Branches allow glycogen to remain soluble, unlike starch.
Whelan’s model
Due to the action of glycogenin, and then of glycogen synthase and branching enzyme, also called glycosyl-(4,6)-transferase (EC 2.4.1.18), glycogen molecule grows exponentially in concentric tiers around the glycogenin core. According to Whelan’s model of glycogen structure, two types of glucose chain can be categorized:
- A-chains, unbranched and present only on the surface;
- B-chains, internal and, on average, with two branching points.
It has been calculated that the maximum size would be of 12 tiers, for a diameter of about 42 nm, a total number of about 55,000 glucose units, and a molecular mass of about 107 kDa.
Moreover, considering that each tier has a thickness of 3.8 nm, assuming glycogen molecule to be a sphere, from the third to the twelfth tier:
- the diameter increases by 5.4 times;
- the volume, which grows according to the cube of its radius, increases 156 times;
- carbohydrate content increases 45.6 fold;
- the number of A-chains in each outermost tier increase exponentially, and is equal to 2n-1, where n corresponds to the number of the tier.
| Level | Diameter (nm) | Chains/Level | Glucose/Level | Total Glucose |
|---|---|---|---|---|
| 1 | — | 1 | 13 | 13 |
| 2 | 3.8 | 3 | 26 | 39 |
| 3 | 7.8 | 7 | 52 | 91 |
| 4 | 11.6 | 15 | 104 | 195 |
| 5 | 15.4 | 31 | 208 | 403 |
| 6 | 19.2 | 63 | 416 | 819 |
| 7 | 23.0 | 127 | 832 | 1651 |
| 8 | 26.8 | 255 | 1664 | 3315 |
| 9 | 30.6 | 511 | 3328 | 6643 |
| 10 | 34.4 | 1023 | 6656 | 13299 |
| 11 | 38.2 | 2047 | 13312 | 26611 |
| 12 | 42.0 | 4095 | 26624 | 53235 |
Considering skeletal muscle, the analysis of the size of glycogen molecules by electron microscopy showed the presence of few full-size particles, and an average diameter of about 25 nm, corresponding to seven tiers.
An important feature of the Whelan’s model is that the outermost tier would contain, in the form of A-chains, about 50 percent of all glucose molecules. This does not mean that these molecules are all accessible to glycogen phosphorylase because the enzyme stalls four residues from the branch point. The intervention of the debranching enzyme, whose activity is slower than glycogen phosphorylase activity, removes the branch and allows glycogenolysis to proceed.
Why is 13th tier not possible? The 13th tier seems to be not possible because of the steric hindrance due to high density of glucose units on the molecule surface. Such a high density of glucose residues would lead to insufficient space for the interaction between the catalytic region of glycogen metabolism enzymes, and then of glycogen synthase, too, and the growing chains.
Furthermore, through mathematical analyzes, it has been suggested that values concerning branch length, about 13 residues in mammals, average branching frequencies per tier, 2, and the maximum number of tiers, 12, are optimal for mobilization of the maximum amount of glucose molecules in the shortest possible time.
Glicogenin
The structure of the glycogen molecule includes the protein glycogenin, which is covalently bonded to the polysaccharide chain. Glycogenin initiates glycogen synthesis by autoglycosylation, catalyzing the addition of 7-11 glucose units to a specific tyrosine residue. This primer chain then acts as substrate for glycogen synthase. In addition, binding to actin filaments, glycogenin anchors the oligosaccharide primer chain to the cytoskeleton.
Phosphate groups
In addition to glycogenin, glycogen molecule covalently binds phosphate groups.
For many years they were considered a contaminant and their amounts were inversely correlated with purity of the sample. Only in the early 1980s they were recognized as an integral part of the polysaccharide, where they seems to be linked to C-2 e C-3 as monoester, probably as a result of a side reaction during the activity of glycogen synthase.
Many studies have suggested that their presence plays a role in regulating glycogen metabolism, similarly to what happens for starch metabolism in plants. Evidence supporting this hypothesis are the identification of laforin, a glycogen phosphatases, and that its mutation is a key factor in Lafora disease, a form of epilepsy characterized, among other things, by an excessive phosphorylation of glycogen.
But how would they act? Several hypotheses have been proposed, and two are reported below.
- It has been suggested that phosphate group, which are hydrophilic, could expose hydrophobic regions, reducing glycogen solubility. The dephosphorylation by laforin, allowing the polysaccharide to remain soluble, would facilitate branch formation.
- Another hypothesis suggests that the degree of phosphorylation would be related to the age of the molecule, acting as a kind quality control. The increase in phosphorylation, which causes a reduction in the solubility of the polysaccharide, would be considered as a metabolic marker that directs it towards the lysosomal degradation, a process called glycophagy, rather than towards glycogenolysis.
Beta granules
Individual glycogen molecules are too small to be detected by light microscopy. Conversely, electron microscopy allowed to identify three types of structures: beta granules, gamma particles, and alpha granules.
Beta granules are made up of the polysaccharide, glycogenin and gamma-particles, which are protein-rich particles of about 3 nm in diameter. Beta granules have a molecular mass of about 106-107 kDa, a diameter of about 20-30 nm, with a rosette-like appearance.
They are considered a rapid energy source.
Under physiological conditions, proteins account for 66-80 percent of their weight. These proteins also bind to each other, to cytoskeleton or to membranes, and are all involved in glycogen metabolism . Some of these are:
- glycogen synthase, the debranching enzyme, and glycogen phosphorylase;
- several regulatory proteins, such as:
- laforin and phosphoprotein phosphatase 1 or PP1 (EC 3.1.3.17);
- phosphorylase kinase (EC 2.7.11.19) and AMPK (EC 2.7.11.31);
- the membrane anchoring protein STDB1; note that phosphorylase kinase binds the membranes, too;
- malin or E3-ubiquitin ligase (EC 2.3.2.27), which binds to glycogen via laforin, and TRIM7.
Unlike the pyruvate dehydrogenase complex or ribosomes, the stoichiometry and the composition of the beta granules is not constant, but rather dynamic, as proteins associate or dissociate from the granule depending on cellular conditions. In addition, differences are observed not only between different cell types but also within the same cell type, for example in skeletal muscle cells depending on different subcellular localizations.
Alpha granules
In the liver, beta granules are organized to form structures called alpha granules.
They are made up of several beta granules, are about 108 kDa in molecular weight, up to about 300 nm in diameter, with a broccoli-like appearance.
Alpha granules are considered a slower energy source than beta granules.
To date, the mechanism underlying their formation is not yet clear, although it seems that beta granules are linked through a protein skeleton rich in disulfide bonds.
Glycogen metabolism: the constructive phase
Glycogen is stored in cell in times of nutritional plenty.
Its synthesis takes place in the cytosol, appears to be associated with actin filament, and can occur from glucose derived from dietary carbohydrates, or from glucose from noncarbohydrate precursors, such as lactate and alanina.
Lactate, produced by skeletal muscle cells working under low oxygen conditions, by red blood cells, that dependent on anaerobic glycolysis for ATP production, and by other tissues, is mostly metabolized in the liver to form glucose via gluconeogenesis. Glucose diffuses from the hepatocytes into the bloodstream and is also transported to the skeletal muscle cells where it may be converted to lactate, thus closing the cycle. This cycle is known as the Cori cycle.
Alanine, a nonessential amino acid, may arise from transamination reactions in which pyruvate produced in glycolysis acts as the acceptor of the amino group of amino acids used for energy. Therefore, alanine is the means by which the carbon skeleton of the conjugate base of pyruvic acid, namely, pyruvate, and amino groups are transported from extrahepatic tissues to the liver, where the carbon skeleton is used to form glucose via gluconeogenesis, and the amino groups is converted into urea through the urea cycle. Glucose diffuses into the bloodstream and reaches the peripheral tissues where it may be converted into pyruvate, thus closing the cycle which is known as the glucose-alanine cycle.
Glucose enters the cells through transmembrane carrier proteins known as glucose transporters (GLUT), of which GLUT4 is primarily expressed in insulin-dependent or insulin-sensitive tissues, such as skeletal and cardiac muscle, liver, and adipose tissue. GLUT4 is the insulin-responsive glucose transporter.
Once in the cell, glucose is phosphorylated to glucose 6-phosphate. This reaction is catalyzed, in hepatocytes and pancreatic beta cells, by glucokinase or hexokinase IV (EC 2.7.1.1), and by other hexokinases in other cell types.
The fate of glucose 6-phosphate depends on the metabolic status of the cell. It may enter the glycolytic pathway to provide energy and/or building blocks for many other metabolic pathways. Alternatively, the isomerization of glucose 6-phosphate to glucose 1-phosphate, an example of position isomerism, may occur, in the reversible reaction catalyzed by phosphoglucomutase (EC 5.4.2.2). Glucose 1-phosphate may be used for glycogen synthesis, or may enter the pentose phosphate pathway, when the reduced coenzyme NADPH is needed for reductive biosynthesis, such as the synthesis of cholesterol and fatty acids, or when ribose 5-phosphate is needed for the synthesis of nucleotides.
When glycogen synthesis predominates, glucose 1-phosphate is converted to UDP-glucose in the reaction catalyzed by UDP-glucose pyrophosphorylase (EC 2.7.7.9), at the expense of one UTP. UDP-glucose is the donor of glucose residues. Initially, glycogenin catalyzes the addition of glucose to the hydroxyl group of Tyr194, after which the enzyme adds 6-10 more glucose residues to form an oligosaccharide chain of 7-11 units. The oligosaccharide chain acts as a substrate for glycogen synthase that, with branching enzyme, leads to glycogen synthesis.
Glycogen metabolism: the degradative phase
Unlike glycogen synthesis, the catabolic or degradative phase of glycogen metabolism occurs both in the cytosol and in the lysosomes, but through different metabolic pathways.
Glycogenolysis is associated with the endoplasmic and sarcoplasmic reticulum, and releases glucose for energy. Reactions catalyzed by glycogen phosphorylase, alpha-(1,4)-glucan-6-glycosyltransferase, and amylo-alfa-(1,6)-glucosidase or debranching enzyme lead to the release of glucose 1-phosphate, for about 90 percent, and free glucose, the remaining 10 percent. In skeletal muscle cell, hexokinase activity is so high that any free glucose molecule is immediately phosphorylated to glucose 6-phosphate, which cannot diffuse out of the cell. In hepatocytes, kidney cortex cells and enterocytes, having the gluconeogenic pathway, glucose 6-phosphatase (EC 3.1.3.9) catalyzes the dephosphorylation of glucose 6-phosphate, resulting from isomerization of glucose 1-phosphate, to glucose that can leave the cell and help regulate blood glucose levels.
Lysosomal degradation
Although glycogen is synthesized in the cytosol, it is also found in lysosomes. Lysosomal glycogen may be the product of an autophagic mechanism, and corresponds to about 10 percent and 5 percent of total glycogen content of the liver and muscle, respectively.
According to one hypothesis, the degree of phosphorylation of glycogen would be one of the key factors in the regulation of its lysosomal metabolism. The degree of phosphorylation would be correlated with the age of the polysaccharide, acting as a quality control system: the increase in phosphorylation, leading to a decrease in solubility of granules, would be considered as a marker that directs glycogen metabolism toward lysosomal degradation, a process also known as glycophagy.
In lysosomes, glycogen breakdown is catalyzed by the enzyme acid alpha-(1,4)-glucosidase (EC 3.2.1.20). The hydrolysis leads to the release of D-glucose. As the enzyme preferably hydrolyzes the α-(1,4) glycosidic bonds, it is not yet clear how the α-(1,6) glycosidic bonds are hydrolyzed.
The importance of the lysosomal catabolism of the polysaccharide is underscored by Pompe disease or type II glycogenosis, due to a mutation of the acid alpha-glucosidase gene. The deficiency of functional acid alpha-(1,4)-glucosidase leads to an overaccumulation of glycogen in lysosomes and vesicular structures. In its most severe form, this glycogenosis is fatal within the first year of life.
Coordinated regulation of glycogen metabolism
Glycogen synthesis and glycogenolysis are exergonic processes, so, if they operate simultaneously, they lead to a waste of energy. In the cell, the two metabolic pathways are under stringent control and are reciprocally regulate so that when one is active, the other slows down. During evolution, this has been achieved by selecting different enzymes to catalyze the key steps of the two pathways, like what occurred for the reciprocal regulation of glycolysis and gluconeogenesis. The key enzymes are glycogen phosphorylase and glycogen synthase, whose activity is regulated through:
- allosteric modifications, occurring on a time scale of milliseconds, are instantly reversible, and involve as effectors calcium ions, glucose, and metabolites that signal the metabolic status of the cell, namely, ATP, AMP and glucose 6-phosphate;
- covalent modifications, that is, phosphorylation and dephosphorylation of specific target proteins such as, in addition to the aforementioned enzymes, phosphorylase kinase, phosphoprotein phosphatase 1, and glycogen synthase kinase 3 (EC 2.7.11.26).
Covalent regulation occur on a time scale of seconds, and is triggered by the binding of hormones, the most important of which are insulin, glucagon and adrenaline (epinephrine), to the corresponding receptors on plasma membrane.
Allosteric and covalent mechanisms are superimposed in the coordinated regulation of glycogen metabolism.
Covalent regulation
The metabolic changes induced by the binding of insulin, glucagon and adrenaline to receptors on plasma membrane of hepatocytes and skeletal muscle cells are summarized below.
Insulin is secreted by pancreatic beta cells in response to high blood glucose levels due to, for example, a meal rich in carbohydrates, and has an anabolic effect. When insulin binds to the receptors on the cell surface of hepatocytes and skeletal muscle cells, it triggers a cascade of reactions that results in the dephosphorylation of:
- glycogen phosphorylase, which is inhibited;
- glycogen synthase, which is activated.
In addition, insulin recruits GLUT4 to plasma membrane of skeletal muscle cells. In this way, glycogen synthesis is stimulated and glycogenolysis is inhibited. This helps to lower blood glucose levels.
Glucagon is secreted by pancreatic alpha cells in response to low blood glucose levels and has an catabolic effect. When glucagon binds to the receptors on the cell surface of hepatocytes, it triggers a cascade of reactions that results in the phosphorylation of:
- glycogen phosphorylase, which is activated;
- glycogen synthase, which is inhibited.
Thereby glycogen synthesis is inhibited and glycogenolysis is stimulated. This helps to raise blood glucose levels.
Adrenaline is secreted by the adrenal glands in response to stimulation of the sympathetic nervous system, and plays a role in the fight-or-flight response. It may be secreted also in response to an high intensity exercise. Like glucagon, it has a catabolic effect as it causes the phosphorylation of:
- glycogen phosphorylase, activating it;
- glycogen synthase, inhibiting it.
However, unlike glucagon, adrenaline acts not only on hepatocytes but also on skeletal muscle cells, binding to alpha-1 and beta-1 adrenergic receptors, and to beta-2 adrenergic receptors, respectively.
Vasopressin and adrenaline, when adrenaline binds to the alpha-1 adrenergic receptors, trigger intracellular pathways leading to the release of calcium ions from the endoplasmic reticulum, and, as previously seen, the stimulation of glycogenolysis and inhibition of glycogen synthesis.
Allosteric regulation
The metabolic changes induced by the binding of the allosteric effectors AMP, ATP, glucose, glucose 6-phosphate, and calcium ions to the respective binding sites on target enzymes are summarized below.
SKELETAL MUSCLE
In response to an increase in AMP concentration:
- phosphorylase kinase is activated;
- glycogen phosphorylase b is activated.
As a result of an increase in ATP concentration:
- phosphorylase kinase is inhibited;
- glycogen phosphorylase b is inhibited.
In response to an increase in glucose 6-phosphate concentration:
- PP1 is activated;
- glycogen phosphorylase b is inhibited;
- the phosphorylated form of glycogen synthase, glycogen synthase b, is activated via a conformational change that favors the dephosphorylation by PP1. This allosteric activation allows glycogen synthase to act as a glucose 6-phosphate sensor.
Therefore, when the cellular concentrations of ATP and glucose 6-phosphate are low and AMP concentration is high, glycogen synthase is inhibited, glycogen phosphorylase is stimulated. Consequently, glycogenolysis is stimulated and glycogen synthesis is inhibited.
Conversely, when the concentrations of ATP and glucose-6-phosphate are high, glycogen synthesis is stimulated and glycogenolysis inhibited.
In skeletal muscle cells, an increase in the intracellular concentration of calcium ions, released from the sarcoplasmic reticulum, triggers muscle contraction. Moreover, calcium ions binds to calmodulin, that is the delta subunit of muscle phosphorylase kinase. Calcium ions-calmodulin activates the kinase that phosphorylates glycogen phosphorylase and glycogen synthase, activating the first and inhibiting the latter.
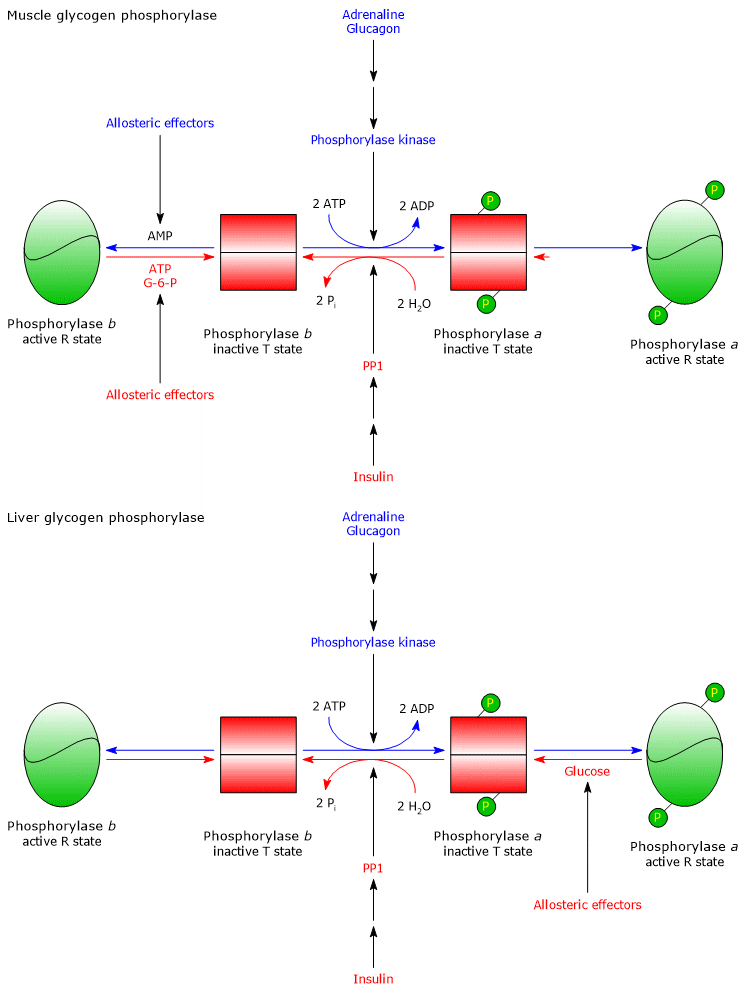
LIVER
In the liver, glycogen phosphorylase b is not activated by AMP, whereas glycogen phosphorylase a is inhibited by an increase in blood glucose levels. How? Glucose concentration in the liver reflects the blood concentration of glucose. In response to an increase in blood glucose levels, glucose binds to an inhibitory allosteric site on glycogen phosphorylase a and triggers a conformational change that exposes phosphorylated serine residues, that are dephosphorylated by PP1, leading to glycogen phosphorylase inactivation. Therefore, glycogen phosphorylase acts as a blood glucose sensor, responding appropriately to its changes.
Where is glycogen found in humans?
In humans, glycogen occurs in all cells, although the main stores are found in the liver and skeletal muscle, where, depending on nutritional status, it can represent up to 10 percent of the liver mass and 2 percent of muscle mass. Therefore, skeletal muscle has a limited capacity to store glycogen than liver. However, as its mass is greater than that of the liver, the muscle content of glycogen is about double than that of the liver. For example, in a non-fasting 70 kg adult male there are about 100 g of glycogen in the liver and about 250 g in the muscle. Athletes can reach higher values, as in best male marathon runners, whose stores in liver and muscle are equal to about 475 g, corresponding to about 1,900 kcal.
The amount of glycogen stored are much lower than those of fats because fats are a form of energy storage much more efficient. Why?
- They can be stored in anhydrous form, whereas the amount of water bound to glycogen is equal to 2-3 times its weight.
- As the stored fats are insoluble in water they are osmotically inert.
- The oxidation of one gram of glycogen yield about 4 kcal, whereas one gram of fat about 9 kcal, then about double the energy.
Why is it important to humans?
Fats, proteins and glycogen are energy stores that the body uses when needed.
In animals, fats are second only to proteins as reserve of energy, although proteins are an energy store of last resort, such as during a prolonged fast.
In a healthy adult subject, body fat accounts for about 21 percent of the body weight in man and 26 percent in woman. This means that, in an adult male weighing 70 kg, body fat is sufficient to cover the body’s energy expenditures for approximately 2 months. Conversely, glycogen stores are sufficient for about one day of the body’s energy expenditures. Nevertheless, glycogen is accumulated. Why?
- Unlike glucose, fatty acids, a class of lipids cannot be metabolized anaerobically, and therefore cannot be used to produce energy by skeletal muscle during anaerobic exercises. However, it should be emphasized that the energy yield of glycogen, or rather of glucose released from glycogen, is different under aerobic and anaerobic conditions. Finally, muscle cannot oxidize fatty acids as quickly as it does with glucose stored in glycogen.
- Animals cannot convert fatty acids into glucose, so they cannot be used to maintain glycemic homeostasis. Although glucose released from muscle glycogen remains within the cell, glucose released from hepatic glycogen, and to a lesser extent from renal glycogen, thanks to the enzyme glucose 6-phosphatase, enter the systemic circulation contributing to the regulation of blood glucose levels.
- Glycogen has a specialized role in fetal lung type II pulmonary cells, or type II pneumocytes, the lung cells susceptible to SARS-CoV-2. At about 26 weeks of gestation they start to accumulate glycogen that serves as major substrate for the synthesis of the lipids of pulmonary surfactant, of which dipalmitoylphosphatidylcholine is the major component.
- The brain contains a small amount of glycogen, too, primarily in astrocytes. It accumulates during sleep and is mobilized upon waking, therefore suggesting its functional role in the conscious brain. These glycogen stores also provide a moderate degree of protection against hypoglycemia.
Glycogen and muscle work
Carbohydrates, namely glucose, and fatty acids are the main energy sources for muscle during exercise, and their relative contribution varies depending on the intensity and duration of exercise, as summarized below:
- <30 percent VO2max: mainly fatty acids;
- 40-60 percent VO2max: fatty acids and carbohydrates;
- 75 percent VO2max: mainly carbohydrates;
- >80 percent VO2max: almost exclusively carbohydrates.
Therefore, the contribution of glycogen to the energy needed to support muscle work increases with increasing exercise intensity, whereas that of fatty acids decreases. Furthermore, when no carbohydrates are ingested, performance is determined by glycogen stores in skeletal muscle and liver, whose relative consumption is different: as the intensity increases, muscle glycogen consumption increases whereas liver glycogen consumption remains more or less constant.
Note: the relative contribution of fatty acids and glycogen as energy sources also varies according to the athlete’s level of training.
Energy yield under anaerobic conditions
Under anaerobic conditions, the oxidation of glucose to lactate via anaerobic glycolysis yields two molecules of ATP.
Below, the yield of ATP from anaerobic oxidation of glucose released during glycogenolysis.
Glycogen phosphorylase and the oxidation of glucose-1-phosphate under anaerobic conditions
Glycogen synthesis from glucose requires 2 ATP for each molecule of glucose.
The release of glucose-1-phosphate by the action of glycogen phosphorylase allows the recovery of one of the 2 molecules of ATP used in the preparatory phase of glycolysis. The yield of anaerobic oxidation of glucose-6-phosphate, generated from glucose-1-phosphate through the action of phosphoglucomutase, is therefore three ATP molecules, not two, because:
- one molecule of ATP, instead of two, is used in the preparatory phase of glycolysis, because hexokinase reaction is bypassed;
- four molecules of ATP are produced in the payoff phase of glycolysis.
Cost-gain rate is 1/3, meaning an energy yield of approximately 66.7 percent.
The reaction is:
Glycogen(n glucose residues) + 3 ADP + 3 Pi → Glycogen(n-1 glucose residues) + 2 Lactate + 3 ATP
By considering the two molecules of ATP used in the synthesis of glycogen and the anaerobic oxidation of glucose-1-phosphate to lactate, there is a yield of one molecule of ATP for each molecule of glucose stored. The resulting reaction is:
Glucose + ADP + Pi → 2 Lactate + ATP
Debranching enzyme and the oxidation of glucose under anaerobic conditions
By considering the glucose released by the action of debranching enzyme, the yield of ATP is zero because:
- two molecules of ATP are used in the synthesis of glycogen from glucose;
- debranching enzyme releases glucose, then, two molecules of ATP will be used in the preparatory phase of glycolysis;
- four molecules of ATP are produced in the payoff phase of glycolysis.
If we now consider the oxidation to lactate of all glucose released from glycogen, there is an energy yield equal to:
1-{[(1/3)*0,9]+[(2/2)*0,1]}=0,60
Then, under anaerobic conditions, there is an energy yield of 60 percent, hence, glycogen is a good storage form of energy.
Energy yield under aerobic conditions
Under aerobic conditions, the oxidation of glucose to CO2 and H2O via glycolysis, pyruvate dehydrogenase complex, Krebs cycle, mitochondrial electron transport chain, and oxidative phosphorylation yields about 30 molecules of ATP.
Below, the yield of ATP from aerobic oxidation of glucose released from glycogen by the action of glycogen phosphorylase and debranching enzyme is considered.
Glycogen phosphorylase and the oxidation of glucose-1-phosphate under aerobic conditions
Oxidation of glucose-6-phosphate, generated from glucose-1-phosphate by the action of phosphoglucomutase, to carbon dioxide and water yields 31 molecules of ATP, and not 30, because only one molecule of ATP is used in the preparatory phase of glycolysis. The cost-to-gain ratio is 1:31, meaning the energy efficiency is approximately 97 percent.
The overall reaction is:
Glycogen(n glucose residues) + 31 ADP + 31 Pi → Glycogen(n-1 glucose residues) + 31 ATP + 6 CO2 + 6 H2O
By considering the two molecules of ATP used in the synthesis of glycogen and the aerobic oxidation of glucose-1-phosphate to carbon dioxide and water, there is a yield of 29 molecules of ATP for each molecule of glucose stored.
The total reaction is:
Glucose + 29 ADP + 30 Pi → 29 ATP + 6 CO2 + 6 H2O
Debranching enzyme and the oxidation of glucose under aerobic condition
By considering the glucose released by the action of debranching enzyme, there is a yield of 30 molecules of ATP, because two molecules of ATP are used in the preparatory phase of glycolysis. The cost-gain rate is 2/30, namely, there is an energy yield of about 93,3 percent.
If we now consider the oxidation to carbon dioxide and water of all glucose released from glycogen, there is an energy yield equal to:
1-{[(1/31)*0,9]+[(2/30)*0,1]}=0,96
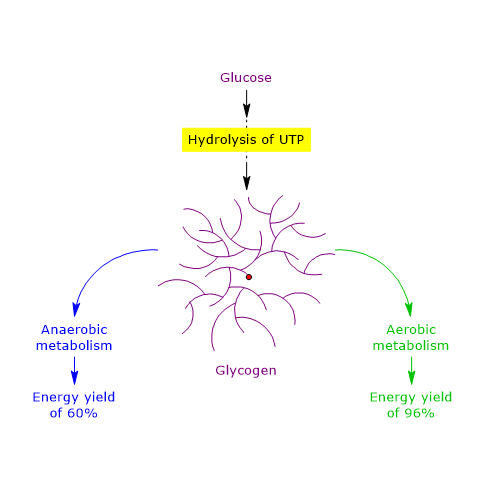 Then, under aerobic conditions, there is an energy yield of 96 percent, hence, glycogen is a extremely efficient storage form of energy, with a gain of 36 percent compared to anaerobic conditions.
Then, under aerobic conditions, there is an energy yield of 96 percent, hence, glycogen is a extremely efficient storage form of energy, with a gain of 36 percent compared to anaerobic conditions.
References
- Adeva-Andany M.M., González-Lucán M., Donapetry-García C., Fernández-Fernández C., Ameneiros-Rodríguez E. Glycogen metabolism in humans. BBA Clin 2016;27;5:85-100. doi:10.1016/j.bbacli.2016.02.001
- Alberts B., Johnson A., Lewis J., Morgan D., Raff M., Roberts K., Walter P. Molecular Biology of the Cell. 6th Edition. Garland Science, Taylor & Francis Group, 2015
- Beelen M., Burke L.M., Gibala M.J., van Loon J.C. Nutritional strategies to promote postexercise recovery. Int J Sport Nutr Exerc Metab 2010:20(6);515-32. doi:10.1123/ijsnem.20.6.515
- Berg J.M., Tymoczko J.L., and Stryer L. Biochemistry. 5th Edition. W. H. Freeman and Company, 2002
- Deng B., Sullivan M.A., Chen C., Li J., Powell P.O., Hu Z., Gilbert R.G. Molecular structure of human-liver glycogen. PLoS ONE 2016;11(3):e0150540. doi:10.1371/journal.pone.0150540
- Fontana J.D. The presence of phosphate in glycogen. FEBS Lett 1980;1:109(1):85-92. doi:10.1016/0014-5793(80)81317-2
- Garrett R.H., Grisham C.M. Biochemistry. 4th Edition. Brooks/Cole, Cengage Learning, 2010
- Gentry M.S., Guinovart J.J., Minassian B.A., Roach P.J., and Serratosa J.M. Lafora disease offers a unique window into neuronal glycogen metabolism. J Biol Chem 2018;293(19):7117-25. doi:10.1074/jbc.R117.803064
- Gunja-Smith Z., Marshall J.J., Mercier C., Smith E.E. and Whelan W.J. A revision of the Meyer-Bernfeld model of glycogen and amylopectin. FEBS Lett 1970:12(2);101-104. doi:10.1016/0014-5793(70)80573-7
- Melendez R., Melendez-Hevia E., and Cascante M. How did glycogen structure evolve to satisfy the requirement for rapid mobilization of glucose? A problem of physical constraints in structure building. J Mol Evol 1997;45(4):446-55. doi:10.1007/PL00006249
- Moran L.A., Horton H.R., Scrimgeour K.G., Perry M.D. Principles of Biochemistry. 5th Edition. Pearson, 2012
- Nelson D.L., M. M. Cox M.M. Lehninger. Principles of biochemistry. 6th Edition. W.H. Freeman and Company, 2012
- Prats C., Graham T.E., and Shearer J. The dynamic life of the glycogen granule. J Biol Chem 2018;293(19):7089-98. doi:10.1074/jbc.R117.802843
- Roach P.J., Depaoli-Roach A.A., Hurley T.D and Tagliabracci V.C. Glycogen and its metabolism: some new developments and old themes. Biochem J 2012;441:763-87. doi:10.1042/BJ20111416
- Rosenthal M.D., Glew R.H. Medical Biochemistry – Human Metabolism in Health and Disease. John Wiley J. & Sons, Inc., 2009
- Shearer J. and Graham T.E. Novel aspects of skeletal muscle glycogen and its regulation during rest and exercise. Exerc Sport Sci Rev 2004;32(3):120-6. doi:10.1097/00003677-200407000-00008
- Stipanuk M.H., Caudill M.A. Biochemical, physiological, and molecular aspects of human nutrition. 3rd Edition. Elsevier health sciences, 2013 [Google eBooks]
- Voet D. and Voet J.D. Biochemistry. 4th Edition. John Wiley J. & Sons, Inc. 2011
- Whelan W.J. Enzymic explorations of the structures of starch and glycogen. Biochem J 1971;122(5):609-622. doi:10.1042/bj1220609