Sweeteners, or sugar substitutes, are additives that, when added to foods and beverages, provide a sweet taste and, in some cases, enhance their flavor, like flavor enhancers.[4][5]
Although they offer sweetness comparable to that of table sugar or sucrose, sweeteners add few calories or no calories, and in this case are categorized as zero-calorie or non-nutritive.[8]
They are used in a wide range of foods and beverages, such as dairy products like yogurt or puddings, canned foods, jams, jellies, soft drinks, powdered drink mixes, chewing gum, candy, and toothpaste, as well as in all products marketed as “diet” or “sugar-free”.
According to EU regulations on food additives, not all sweet-tasting substances are classified as sweeteners.[4] Examples include monosaccharides, such as glucose and fructose, and disaccharides, such as sucrose, lactose, maltose, and trehalose, as well as oligosaccharides. Foods containing these sugars, such as honey, invert sugar, molasses, maple syrup, corn syrup, and certain fruit purees, that are used for their sweetening properties, are also excluded.[5][14]
Their presence in products is indicated on the label’s ingredient list, where they are identified by name and/or E number, like other food additives potentially present.
They are commercially available in various forms, such as sachets, small pills, powder, or liquid.
Based on the scientific evidence currently available, their use in limited amounts is not harmful to human health. However, like other food additives, sweeteners are also subject to regular safety reviews.[9]
Contents
Natural and artificial sweeteners
Sweeteners can be extracted from plants, produced using microorganisms, or synthesized.
Examples of plant-derived sweeteners include thaumatin (E 957), a mixture of closely related proteins extracted from the fruit of Thaumatococcus daniellii, and steviol glycosides (E960a), which are extracted from the leaves of Stevia rebaudiana. Neohesperidin (E959) is obtained by hydrogenation of a flavone glucoside present in the flowers, fruit and peel of grapefruit and bitter orange.[3][6]
Erythritol (E968) is produced by bacterial fermentation of plant sugars.
Finally, other sweeteners, such as saccharin (E954), are synthesized artificially.
Sweetness power
The sweetness intensity of sweeteners, compared to sucrose, varies greatly from one product to another.
Some sweeteners, such as polyols, for example sorbitol (E420), erythritol, and xylitol (E967), have a sweetness slightly lower or equal to that of sucrose. Others, such as cyclamates (E952), are dozens of times sweeter, while some are hundreds of times sweeter, such as aspartame (E951), acesulfame K (E950), saccharin, and sucralose (E955). Some, like neohesperidin, thaumatin, and neotame (E961), are even thousands of times sweeter than table sugar.[13]
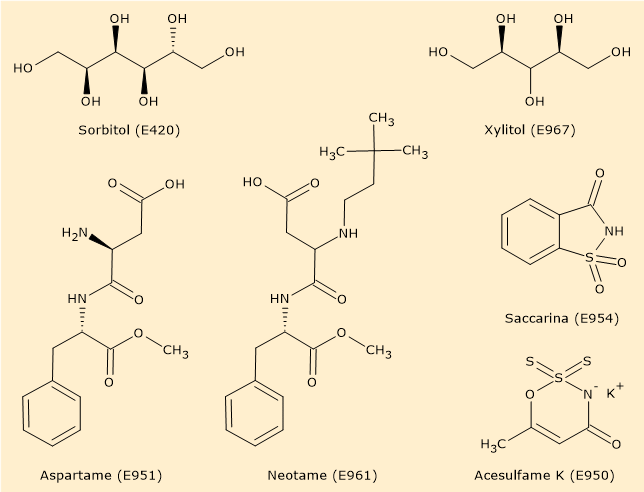 Obviously, the higher the sweetness power of the sweetener, the smaller the amount required. As a result, their caloric contribution is negligible.
Obviously, the higher the sweetness power of the sweetener, the smaller the amount required. As a result, their caloric contribution is negligible.
Examples of sweeteners
Below is a review of the sweeteners included in the list of food additives approved in the European Community according to Regulation 1129/2011 of the European Commission, published on November 11, 2011. This list is an amendment of Annex II to Regulation (EC) No. 1333/2008 of the European Parliament, and was in turn amended in 2013.[4]
| E-number | Additive | Sweetness compared to sucrose |
| E420 | Sorbitol | 0.5-1 |
| E421 | Mannitol | 0.7 |
| E950 | Acesulfame K | 200 |
| E951 | Aspartame | 180-200 |
| E952 | Ciclamate | 30 |
| E953 | lsomalt | 0,5 |
| E954 | Saccharin | 300 |
| E955 | Sucralose | 600 |
| E957 | Thaumatin | 2,000-3,000 |
| E959 | Neohesperidine DC | 1900 |
| E960a | Steviol glycosides from Stevia |
200-400 |
| E960b | Enzymatically produced steviol glycosides |
200-400 |
| E960c | Glucosylated steviol glycosides | 200-400 |
| E961 | Neotame | 7,000-13,000 |
| E962 | Salt of aspartame-acesulfame | 350 |
| E964 | Polyglycitol syrup | 0.4-0.9 |
| E965 | Maltitol | 1 |
| E966 | Lactitol | 0.5 |
| E967 | Xylitol | 1 |
| E968 | Erythritol | 0.6-0.8 |
| E969 | Advantame | 20,000 |
There are differences between the sweeteners authorized in the European Community and those authorized in the United States, only six, or in Great Britain, which allows only eight.[7][13]
Health effects
Based on available scientific evidence, the sweeteners currently in use are safe for the general population, including pregnant women.[9] However, some should be limited or avoided in certain health conditions.
Aspartame should be avoided by individuals with phenylketonuria, as it is a source of phenylalanine, an amino acid that these individuals cannot metabolize.
Polyols, which are naturally present in many fruits and vegetables, can have a laxative effect if consumed in large amounts. If they make up more than 10 percent of a product, the label must indicate that excessive consumption may have laxative effects.[7] Reducing polyol intake is one of the recommendations of the low FODMAPs diet, often recommended to individuals with irritable bowel syndrome.[11]
Studies that have attempted to link sweetener use with weight loss and the fight against obesity have given contradictory results, while there is no evidence that they increase appetite.[10][12]
As a precaution, sweeteners should not be given to children under two years of age.[2]
Since a high sugar consumption increases the risk of tooth decay, the use of sweeteners, provided that the food contains no sugar, reduces the risk.[7]
It is important to emphasize that sweeteners do not cause cancer.[1]
References
- ^ Artificial sweeteners and cancer. National Cancer Institute. https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/artificial-sweeteners-fact-sheet
- ^ Baker-Smith C.M., de Ferranti S.D., Cochran W.J. COMMITTEE ON NUTRITION, SECTION ON GASTROENTEROLOGY, HEPATOLOGY, AND NUTRITION. The Use of Nonnutritive Sweeteners in Children. Pediatrics 2019;144(5):e20192765. doi:10.1542/peds.2019-2765
- ^ Borrego F. and Montijano H. Neohesperidin Dihydrochalcone. In: Nabors LO (ed). Alternative Sweeteners. Marcel Dekker, Inc, New York, USA, 2001. pp. 85–99.
- ^ a b c Commission Regulation (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. https://eur-lex.europa.eu/eli/reg/2011/1129/2013-11-21
- ^ a b EFSA. Sweeteners. Last reviewed date: 21 March 2024.
- ^ Fry J.C. 3 – Natural low-calorie sweeteners. Editor(s): David Baines, Richard Seal. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Natural Food Additives, Ingredients and Flavourings. Woodhead Publishing. 2012:41-75. doi:10.1533/9780857095725.1.41
- ^ a b c The truth about sweeteners. Page last reviewed: 20 February 2023 https://www.nhs.uk/live-well/eat-well/food-types/are-sweeteners-safe/
- ^ Nutritive and Nonnutritive Sweetener Resources | Food and Nutrition Information Center | NAL | USDA”. Archived from the original on 22 September 2020. Retrieved 17 September 2020.
- ^ a b Rios-Leyvraz M. and Montez J. Health effects of the use of non-sugar sweeteners: a systematic review and meta-analysis. World Health Organization 2022. https://www.who.int/publications/i/item/9789240046429
- ^ Roberts J.R. The paradox of artificial sweeteners in managing obesity. Curr Gastroenterol Rep 2015;17(1):423. doi:1007/s11894-014-0423-z
- ^ Shepherd S.J., Parker F.C., Muir J.G., Gibson P.R. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol 2008;6:765-71.doi:1016/j.cgh.2008.02.058
- ^ Te Morenga L., Mallard S., Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2012;346:e7492. doi:1136/bmj.e7492
- ^ a b U.S. Food and Drug Administration. Aspartame and other sweeteners in food. Content current as of: 09/25/2024 https://www.fda.gov/food/food-additives-petitions/aspartame-and-other-sweeteners-food
- ^ Ziesenitz S.C. Authorised EU health claim for fructose in foods, nutrients and food ingredients with authorised EU health claims: Volume 2, 2015 doi:1016/B978-1-78242-382-9.00011-6