Short-chain fatty acids (SCFAs) are saturated fatty acids with a straight or branched carbon chain made of 2–5 carbon atoms, and are acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid, and 2-methylbutyric acid.[1]
In humans, they are, along with secondary bile salts, the main metabolites produced by bacteria in the gut microbiota in the cecum and colon, and derive almost entirely from the anaerobic fermentation of non-digestible carbohydrates.[2] The most abundant are acetic acid, propionic acid, and butyric acid, which represent 90–95% of the produced SCFAs. The remaining percentage is made of the branched SCFAs.[3]
They are the major anions present in the colon.[4] Their concentration is higher in the cecum and in the proximal colon than in the distal part, where the substrates for their synthesis are depleted.[5][6][7] They are able to reduce the colonic pH and thus acidify the stool.
About 90–95% of the SCFAs are absorbed in the cecum and colon, whereas 5–10% are excreted with the feces, and are thought to provide about 70% of the energy needs of colonocytes.[8][9]
Short-chain fatty acids are able to modulate the physiology and composition of the gut microbiota. Furthermore, a growing body of research suggests that they play an important role in maintaining human health.[4]
Contents
- Sources
- Properties
- Health effects
- Synthesis
- Membrane receptors
- Absorption
- Role in colonocytes
- Transport
- Hepatic and extrahepatic metabolism
- References
Sources
Like medium-chain fatty acids and long-chain fatty acids, short-chain fatty acids are present in animal and plant tissues mostly in the form of triglycerides, although in much lower amounts than long-chain ones.[10]
In adults, the main food source is milk and dairy products, where butyric acid is the SCFA present with the highest concentration. Other sources are some vegetable oils, such as palm kernel oil and coconut oil.
In breastfed infants, the main source is breast milk.[11]
However, for humans, and most mammals, the most important source is the anaerobic fermentation of fibers and resistant starch, namely, indigestible carbohydrates, by the bacteria of the gut microbiota.[12] Approximately 500–600 mM of SCFAs are produced through this pathway per day. Acetic, propionic, and butyric acids are present in a molar ratio of about 60:20:20, respectively, although the relative proportion of each depends on the microbiota composition, the substrate, and the intestinal transit time.[2][4]
Properties
Short-chain fatty acids have carbon chains made of 2–5 carbon atoms, a characteristic that strongly affects their physical properties.
Acetic acid, propionic acid, butyric acid, and valeric acid are straight-chain fatty acids, whereas isobutyric acid, isovaleric acid, and 2-methylbutyric acid are branched-chain fatty acids.[13]
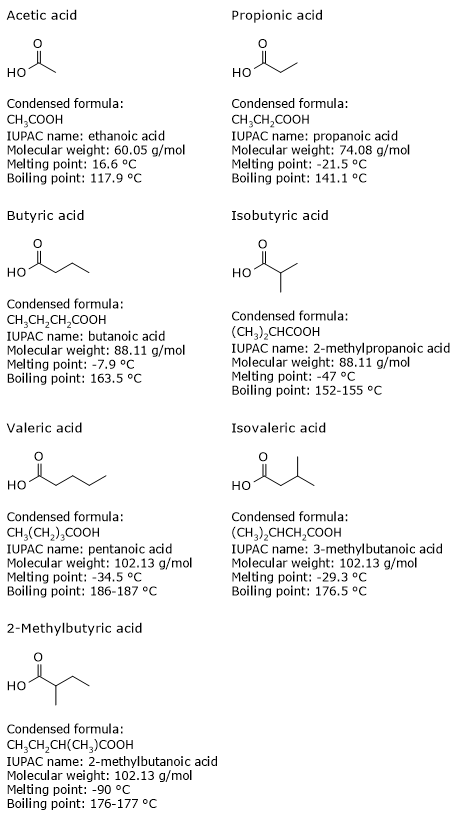
They are small molecules, and are the smallest among all lipids.
They are liquid at room temperature, and are soluble in polar solvents such as water, unlike saturated fatty acids with longer carbon chains, for which solubility in polar solvents, considering those with straight chain, decreases as the length of the chain increases, as the hydrophobic part of the molecule is the carbon chain, whereas the carboxyl group is polar.[11]
Finally, it should also be noted that butyric acid and isobutyric acid, which have the molecular formula C4H8O2, are an example of chain isomerism, as are valeric acid, isovaleric acid, and 2-methylbutyric acid, which have the molecular formula C5H10O2.
Health effects
Short-chain fatty acids appear to play a crucial role in maintaining human health.[7] Their activity seems to occur through direct and/or indirect effects on cellular processes such as proliferation, differentiation, and gene expression, thus contributing to the regulation of processes such as glucose homeostasis, intestinal and immune function, and the regulation of the gut-brain axis.[14]
Their health effects seem to be confirmed by studies showing that intestinal dysbiosis appears to be implicated in metabolic pathologies, such as disorders involving glucose homeostasis, and behavioral and neurological pathologies, such as depression, and Alzheimer’s and Parkinson’s.[15]
Synthesis
In humans, the enzyme equipment carrying out carbohydrate digestion lacks the enzymes capable of digesting fiber and resistant starch, the latter so-called precisely because it resists the action of alpha-amylase.
On the contrary, the bacteria of the gut microbiota encode a large number of different glycoside hydrolases, more than 260, which also hydrolyze fibers and resistant starch, releasing the constituent monosaccharides.[16]
Hexoses and deoxyhexoses enter glycolysis, and pentoses enter the pentose phosphate pathway, to give pyruvate, which is the main precursor for the synthesis of short-chain fatty acids.[17][18][19]
The synthesis of SCFAs is affected by several factors; below are some examples.
- The fiber content of the diet. For example, a diet rich in fibers, such as the Mediterranean diet, may influence their synthesis.[2]
- The composition of the gut microbiota.[20]
- The pH of the intestinal lumen, as the bacteria that produce butyric acid dominate at a pH value around 5.5, while the bacteria that produce acetic and propionic acids dominate at a pH value around 6.5.[21]
- The gut transit time.[22]
- The amount of oxygen in the intestinal lumen.[6]
Acetic acid and propionic acid are mainly produced by species of the phylum Bacteroidetes, while butyric acid, for whose synthesis resistant starch is particularly important, by species of the phylum Firmicutes.[23][24]
Synthesis of acetic acid
Acetic acid, the most abundant SCFA in the colon, can be synthesized via the Wood-Ljungdahl pathway in the reductive direction, through the reduction of CO2 to acetate, or from acetyl-CoA, which is the most important metabolic pathway, responsible for the production of about two thirds of the butyric acid present in the intestinal lumen.[25]
Synthesis of propionic acid
Propionic acid can be synthesized through three different metabolic pathways: the acrylate and succinate pathways, which use lactic acid produced by other bacteria, and the propanediol pathway, in which the precursors are deoxyhexoses.[6]
The acrylate pathway converts lactic acid to propionyl-CoA, via lactoyl-CoA. In the final step, propionyl-CoA is hydrolyzed to propionic acid.[26]
In the succinate pathway, lactate is reduced to pyruvate, which is carboxylated to oxaloacetate, which, through a pathway that has malate, fumarate, succinate, and methylmalonyl-CoA as intermediates, is converted to propionyl-CoA, which in turn is hydrolyzed to propionic acid. The succinate pathway is thought to be the dominant pathway for propionic acid synthesis in the gut.[27]
In the propanediol pathway, some deoxyhexoses, such as fucose and rhamnose, are converted via 1,2-propanediol to propionyl-CoA and then propionic acid.[28]
Synthesis of butyric acid
The synthesis of butyric acid can follow two routes.[4][11]
In most butyric acid-producing bacteria, the short-chain fatty acid is synthesized through a pathway that begins with the condensation of two acetyl-CoA molecules to acetoacetyl-CoA, which, through a pathway that has β-hydroxybutyryl-CoA and crotonyl-CoA as intermediates, is converted to butyryl-CoA. The final step is the release of butyric acid from butyryl-CoA.[29]
In a small number of bacterial species, butyryl-CoA is converted to butyryl phosphate, from which butyric acid is released.[6]
Synthesis from amino acids
Acetic acid, propionic acid, and butyric acid can also be produced from amino acids obtained from peptide and protein degradation, although the amount produced by these pathways is small.[24]
These syntheses occur in the distal part of the colon, often by non-commensal bacteria, as in the case of glutamate and lysine fermentation.[27] Different short-chain fatty acids are produced by the metabolism of different amino acids; below are some examples.[30]
- Glutamic acid mainly produces acetic acid and butyric acid.
- Aspartic acid mainly produces acetic acid and propionic acid.
- The basic amino acids lysine, arginine, and histidine produce acetic acid and butyric acid.
- Cysteine produces acetic, propionic, and butyric acids.
- Methionine mainly produces propionic acid and butyric acid.
- Branched-chain fatty acids derive from the branched-chain amino acids leucine, isoleucine, and valine.
The pH of the intestinal lumen influences the metabolism of proteins by the gut microbiota; for example, their breakdown into amino acids is more likely at neutral or weakly alkaline pH.[31]
It should be noted that potentially toxic compounds, such as ammonia, sulfites and phenols, are also produced from the intestinal metabolism of amino acids.[30]
Endogenous synthesis
Mammals, and therefore humans, have the enzymatic equipment for the endogenous synthesis of short-chain fatty acids. The synthesis occurs mainly in the liver, by β-oxidation cycles which lead to the formation of acyl-CoA with a shorter carbon chain than the starting fatty acid. The acyl-CoA is then hydrolyzed to fatty acid and CoA by an acyl-CoA thioesterase (EC 3.1.2.20).[11][32]
Membrane receptors
Short-chain fatty acids can bind to specific receptors on the plasma membrane, namely G protein-coupled receptors, including GPR41, GPR43, and GPR109A.[33]
The effects produced by the binding of SCFAs on the receptors depend on the cell type. For example, binding to receptors on intestinal L cells is associated with the release of glucagon-like peptide-1 (GLP-1), and peptide YY, hormones that affect appetite and food intake.[4] Binding to enterochromaffin cells induces the release of serotonin, which may affect intestinal motility.[34] Finally, binding to receptors on pancreatic beta-cells increases insulin release.[35]
The different short-chain fatty acids have different abilities to activate the receptors: GPR43 is more likely to be activated by acetic and propionic acids, GPR41 by propionic and butyric acids, while GPR109A is activated by butyric acid.[36]
Absorption
About 90% of the short-chain fatty acids present in the intestinal lumen are absorbed by colonocytes.[37] The passage across the plasma membrane can occur by passive diffusion or by active transport mediated by two types of membrane transporters: the H+-dependent and Na+-dependent monocarboxylate transporters, or MCTs and SMCTs, respectively.[38]
Passive transport affects protonated forms of SCFAs, so it is influenced by the colonic pH value. A weak acidification of the intestinal lumen, which can be due to the metabolic activity of the microorganisms, increases the prevalence of the protonated form and therefore ehances passive transport.[11]
Role in colonocytes
In colonocytes, short-chain fatty acids have both energy and regulatory functions.[33]
When used for energy purposes, acetic acid and butyric acid are converted to acetyl-CoA, and propionic acid to propionyl-CoA. Through the production of ATP, SCFAs contribute to the maintenance of cellular homeostasis, but also, for example, to the maintenance of the integrity of the tight junctions at the cell apex, and therefore of the integrity of the intestinal barrier.[39] Of the three major short-chain fatty acids produced by the gut microbiota, butyric acid is the major source of energy for colonocytes, while acetic and propionic acids are poorly metabolized and mostly drained by the portal vein.[27][40]
Considering the regulatory role, SCFAs are, for example, capable of inhibiting histone deacetylases (EC 3.5.1.98), enzymes that catalyze the removal of acetyl groups from lysine residues of histone proteins, acetyl groups previously inserted by histone acetyltransferase (EC 2.3.1.48).[41] The R groups of deacetylated lysines have positive charges, which allows histone proteins to wrap the DNA more tightly, making the nucleosome more compact and thereby hindering transcription and gene expression. The different short-chain fatty acids have different abilities to inhibit histone deacetylases:
- up to 80% for butyric acid;
- up to 60% for propionic acid;
- acetic acid has the lowest inhibitory rate.[7]
This mode of action on histone deacetylases has been observed not only in the gut and associated immune tissue, but also in the central and peripheral nervous systems.[42]
Transport
Short-chain fatty acids that have not been utilized by colonocytes leave the cell by passive diffusion and active transport across the basolateral membrane, and enter the portal circulation where acetic acid reaches the highest concentration, about 260 mM/L, while propionic and butyric acids reach concentrations of about 30 mM/L.[4]
In the rectum, a small amount of these lipids can pass directly into the systemic circulation, thus bypassing the liver, via the internal iliac vein.[40]
Unlike long-chain fatty acids, short- and medium-chain fatty acids are present in the circulation in free form, namely, as non-esterified fatty acids, and, bound to albumin, reach the liver. Subsequent cell uptake and intracellular transport do not require fatty acid transport proteins, plasma membrane fatty acid translocases, or cytosolic fatty acid binding proteins. Therefore, their oxidation may be much faster than that of long-chain fatty acids and the longest members of medium-chain fatty acids, namely, fatty acid with carbon chains longer than 8 carbon atoms.[11]
Hepatic and extrahepatic metabolism
The liver is an important site for short-chain fatty acid metabolism.[37]
It can absorb about 40% of the acetic acid and 80% of the propionic acid from the portal vein.[43] Propionic acid is mostly metabolized in the liver, where it can also be used as a substrate for gluconeogenesis.[44]
A small amount of the gut-derived SCFAs, about 36% for acetic acid, 9% for propionic acid, and only 2% for butyric acid, reach, through the systemic circulation, the peripheral tissues. In muscle, acetic acid can be used for lipid synthesis or be oxidized for energy production. Furthermore, it is thought that SCFA concentrations in the systemic circulation, even if small, are capable of influencing the metabolism and physiology of peripheral cells and tissues.[4][12]
References
- ^ Cummings J.H. Short chain fatty acids in the human colon. Gut 1981;22(9):763-79. doi:10.1136/gut.22.9.763
- ^ a b c Silva Y.P., Bernardi A., Frozza R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne) 2020;11:25. doi:10.3389/fendo.2020.00025
- ^ Chen X.F., Chen X., Tang X. Short-chain fatty acid, acylation and cardiovascular diseases. Clin Sci (Lond) 2020;134(6):657-676. doi:10.1042/CS20200128
- ^ a b c d e f g Portincasa P., Bonfrate L., Vacca M., De Angelis M., Farella I., Lanza E., Khalil M., Wang D.Q.-H., Sperandio M., Di Ciaula A. Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int J Mol Sci 2022;23:1105. doi:10.3390/ijms23031105
- ^ Abdul Rahim M.B.H., Chilloux J., Martinez-Gili L., Neves A.L., Myridakis A., Gooderham N., Dumas M.-E. Diet-induced metabolic changes of the human gut microbiome: importance of short-chain fatty acids, methylamines and indoles. Acta Diabetol 2019;56:493-500. doi:10.1007/s00592-019-01312-x
- ^ a b c d Deleu S., Machiels K., Raes J., Verbeke K., Vermeire S. Short chain fatty acids and its producing organisms: an overlooked therapy for IBD? EBioMedicine 2021;66:103293. doi:10.1016/j.ebiom.2021.103293
- ^ a b c Liu X.F., Shao J.H., Liao Y.T., Wang L.N., Jia Y., Dong P.J., Liu Z.Z., He D.D., Li C., Zhang X. Regulation of short-chain fatty acids in the immune system. Front Immunol 2023;14:1186892. doi:10.3389/fimmu.2023.1186892
- ^ McLoughlin R.F., Berthon B.S., Jensen M.E., Baines K.J., Wood L.G. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: a systematic review and meta-analysis. Am J Clin Nutr 2017;106(3):930-945. doi:10.3945/ajcn.117.156265
- ^ Brahe L.K., Astrup A., Larsen L.H. Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases? Obes Rev 2013;14(12):950-9. doi:10.1111/obr.12068
- ^ Marten B., Pfeuffer M., Schrezenmeir J. Medium-chain triglycerides. Int Dairy J 2006;16:11, Pages 1374-1382. doi:10.1016/j.idairyj.2006.06.015
- ^ a b c d e f Schönfeld P., Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res 2016;57(6):943-54. doi:10.1194/jlr.R067629
- ^ a b Cummings J.H., Pomare E.W., Branch W.J., Naylor C.P., Macfarlane G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987;28(10):1221-7. doi:10.1136/gut.28.10.1221
- ^ PubChem
- ^ Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol 2019;16(8):461-478. doi:10.1038/s41575-019-0157-3
- ^ Borre Y.E., O’Keeffe G.W., Clarke G., Stanton C., Dinan T.G., Cryan J.F. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 2014;20(9):509-18. doi:10.1016/j.molmed.2014.05.002
- ^ Usuda H., Okamoto T., Wada K. Leaky gut: effect of dietary fiber and fats on microbiome and intestinal barrier. Int J Mol Sci 2021;22(14):7613. doi:10.3390/ijms22147613
- ^ Hugenholtz F., Mullaney J. A., Kleerebezem M., Smidt H., Rosendale D. I. Modulation of the microbial fermentation in the gut by fermentable carbohydrates. Bioact Carbohydr Diet Fibre 2013;2(2)-133-142. doi:10.1016/j.bcdf.2013.09.008
- ^ Krautkramer K.A., Fan J., Bäckhed F. Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol 2021;19(2):77-94. doi:10.1038/s41579-020-0438-4
- ^ Miller T.L., Wolin M.J. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl Environ Microbiol 1996;62(5):1589-92. doi:10.1128/aem.62.5.1589-1592
- ^ Bergman E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev 1990;70(2):567-90. doi: 10.1152/physrev.1990.70.2.567
- ^ Topping D.L., Clifton P.M. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 2001;81(3):1031-64. doi:10.1152/physrev.2001.81.3.1031
- ^ Macfarlane S., Macfarlane G.T. Regulation of short-chain fatty acid production. Proc Nutr Soc 2003;62:67–72. doi:10.1079/PNS2002207
- ^ Louis P., Flint H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 2009;294(1):1-8. doi: 10.1111/j.1574-6968.2009.01514.x
- ^ a b Louis P., Flint H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol 2017;19(1):29-41. doi:10.1111/1462-2920.13589
- ^ Ragsdale S.W., Pierce E. Acetogenesis and the Wood-Ljungdahl pathway of CO2, fixation. Biochim Biophys Acta 2008;1784(12):1873-98. doi:10.1016/j.bbapap.2008.08.012
- ^ Hetzel M., Brock M., Selmer T., Pierik A.J., Golding B.T., Buckel W. Acryloyl-CoA reductase from Clostridium propionicum. An enzyme complex of propionyl-CoA dehydrogenase and electron-transferring flavoprotein. Eur J Biochem 2003;270(5):902-10. doi:10.1046/j.1432-1033.2003.03450.x
- ^ a b c Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 2016;165(6):1332-1345. doi:10.1016/j.cell.2016.05.041
- ^ Scott K.P., Martin J.C., Campbell G., Mayer C.D., Flint H.J. Whole-genome transcription profiling reveals genes up-regulated by growth on fucose in the human gut bacterium Roseburia inulinivorans. J Bacteriol 2006;188(12):4340-9. doi:10.1128/JB.00137-06
- ^ Pryde S.E., Duncan S.H., Hold G.L., Stewart C.S., Flint H.J. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett 2002;217(2):133-9. doi:10.1111/j.1574-6968.2002.tb11467.x
- ^ a b Smith E.A., Macfarlane G.T. Dissimilatory amino acid metabolism in human colonic bacteria. Anaerobe 1997;3(5):327-37. doi:
- ^ Windey K., De Preter V., Verbeke K. Relevance of protein fermentation to gut health. Mol Nutr Food Res 2012;56(1):184-96. doi:10.1002/mnfr.201100542
- ^ Trefely S., Lovell C.D., Snyder N.W., Wellen K.E. Compartmentalised acyl-CoA metabolism and roles in chromatin regulation. Mol Metab 2020;38:100941. doi:10.1016/j.molmet.2020.01.005
- ^ a b Parada Venegas D., De la Fuente M.K., Landskron G., González M.J., Quera R., Dijkstra G., Harmsen H.J.M., Faber K.N., Hermoso M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol 2019;10:1486. doi:10.3389/fimmu.2019.00277
- ^ Fukumoto S., Tatewaki M., Yamada T., Fujimiya M., Mantyh C., Voss M., Eubanks S., Harris M., Pappas T.N., Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol 2003;284(5):R1269-76. doi:10.1152/ajpregu.00442.2002
- ^ Puddu A., Sanguineti R., Montecucco F., Viviani G.L. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediators Inflamm 2014;2014:162021. doi:10.1155/2014/162021
- ^ Brown A.J., Goldsworthy S.M., Barnes A.A., Eilert M.M., Tcheang L., Daniels D., Muir A.I., Wigglesworth M.J., Kinghorn I., Fraser N.J., Pike N.B., Strum J.C., Steplewski K.M., Murdock P.R., Holder J.C., Marshall F.H., Szekeres P.G., Wilson S., Ignar D.M., Foord S.M., Wise A., Dowell S.J. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 2003;278(13):11312-9. doi:10.1074/jbc.M211609200
- ^ a b Xiong R.G., Zhou D.D., Wu S.X., Huang S.Y., Saimaiti A., Yang Z.J., Shang A., Zhao C.N., Gan R.Y., Li H.B. Health benefits and side effects of short-chain fatty acids. Foods 2022;11(18):2863. doi:10.3390/foods11182863
- ^ Kim C.H., Park J., Kim M. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw 2014;14(6):277-88. doi:10.4110/in.2014.14.6.277
- ^ Chambers E.S., Preston T., Frost G., Morrison D.J. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr Nutr Rep 2018;7(4):198-206. doi:10.1007/s13668-018-0248-8
- ^ a b van der Beek C.M., Dejong C.H.C., Troost F.J., Masclee A.A.M., Lenaerts K. Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr Rev 2017;75(4):286-305. doi:10.1093/nutrit/nuw067
- ^ Marchix J., Goddard G., Helmrath M.A. Host-gut microbiota crosstalk in intestinal adaptation. Cell Mol Gastroenterol Hepatol 2018;6(2):149-162. doi:10.1016/j.jcmgh.2018.01.024
- ^ Stilling R.M., van de Wouw M., Clarke G., Stanton C., Dinan T.G., Cryan J.F. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int 2016;99:110-132. doi:10.1016/j.neuint.2016.06.011
- ^ Chambers E.S. Gut-derived short-chain fatty acids: a friend or foe for hepatic lipid metabolism? Nutr Bull 2019;44:154-159. doi:10.1111/nbu.12377
- ^ Leung C., Rivera L., Furness J.B., Angus P.W. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol 2016;13(7):412-25. doi:10.1038/nrgastro.2016.85