A futile cycle, or substrate cycle, occurs when two non-equilibrium opposing reactions, catalyzed by different enzymes, or two opposite metabolic pathways run simultaneously with no other overall effect than the dissipation of energy.[1]
The name of futile cycle was coined because apparently these cycles seemed to confer no benefit to the cell, a sort of metabolic imperfection leading to energy expenditure.[2]
However, recently, they have been recognized as important for the generation of heat, the amplification of metabolic signals, the redistribution of the energy, in form of triglycerides, between adipocytes and hepatocytes, and the modification of fatty acids stored as triglycerides in adipose tissue.
In order to avoid uncontrolled dissipation of energy, futile cycles are strictly regulated.[3][4][5]
Examples of futile cycles are glycolysis and gluconeogenesis when they proceed simultaneously at a high rate in the same cell, the Cori cycle, and the triglyceride/fatty acid cycle.[6]
Contents
- Signal amplification
- Regulation
- Glycolysis and gluconeogenesis
- Cori cycle
- Triglyceride/fatty acid cycle
- Generation of heat
- References
Signal amplification
Let us consider the conversion of A into B, which proceeds at a rate of 100, and B into A, which proceeds at a rate of 90. This results in a net flow of 10. Suppose that an effector increases the rate of conversion of A into B by 30 percent, to 130 percent, and reduces the rate of conversion of B into A by 30 percent, to 63 percent. The resulting net flux is equal to 130-63 = 67, namely, a 30 percent change in the rates of the opposing reactions has led to a 570 percent increase in the net flux.
A mechanism of this type could, at least in part, explain the even 1000-fold increase in carbon flux down the glycolysis in the initial phase of intense exercise.[2]
Regulation
In the course of evolution, the selection of different enzymes to catalyze irreversible and opposing reactions has made possible to avoid or put under strict control futile cycles. How? The selection of one enzyme that catalyzes the conversion of A into B, and another enzyme that catalyzes the opposing reaction, whose activities are regulated separately, allows the control of the net flux.[7]
Enzymatic activities are controlled by:
- allosteric mechanisms;
- covalent modifications;
- modifications in the concentration of the enzymes, due to variations in the ratio between their synthesis and/or degradation. A different mechanism regulates hepatic glucokinase (EC 2.7.1.2). During fasting, the enzyme is reversibly bound to GKPR, one of the liver-specific proteins, which anchors it inside the nucleus, separating it from the other glycolytic enzymes, and thus preventing the futile cycle between glycolysis and gluconeogenesis.
In this way, it is possible to obtain a coordinated regulation of the two opposing pathways, thus avoiding an uncontrolled futile cycle. Obviously, such a fine regulation could not be achieved if a single enzyme would operate in both directions.[8]
Glycolysis and gluconeogenesis
If glycolysis, which converts glucose into pyruvate, the conjugate base of pyruvic acid, with the production of ATP, and gluconeogenesis, which converts pyruvate into glucose with the consumption of ATP, run simultaneously at high rate in the same cell, the net result would be a net consumption of ATP, therefore a futile cycle. This is avoided by the control of the irreversible steps of the two metabolic pathways, in particular the reactions catalyzed by phosphofructokinase-1 (PFK-1; EC 2.7.1.11), and by fructose-1,6-bisphosphatase (FBPase; EC 3.1.3.11), mainly by the allosteric effector fructose 2,6-bisphosphate.[7]
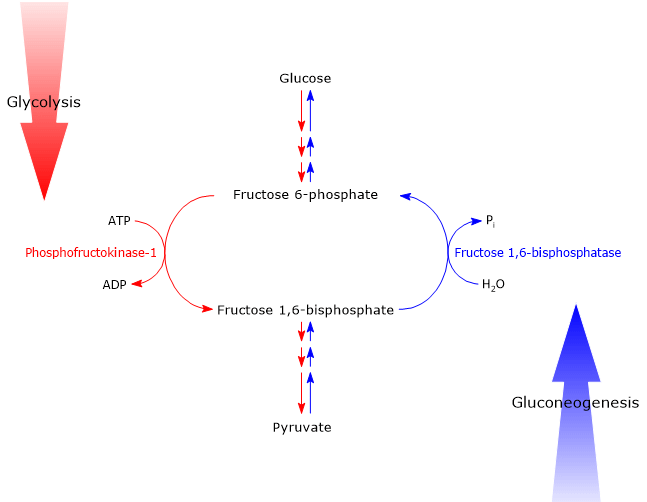
It should be noted that in glycolysis, the control involves all the irreversible reactions, whereas in gluconeogenesis, the key regulatory points are the reactions catalyzed by pyruvate carboxylase (EC 6.4.1.1) and fructose 1,6-bisphosphatase.[8]
Cori Cycle
In the Cori cycle, lactic acid produced from glucose in the muscle and other extrahepatic tissues reaches the liver, where it is converted back into glucose, which, released into the circulation, returns to the muscle and other extrahepatic tissues, thereby closing the cycle. From an energetic point of view, the Cori cycle can be considered a futile cycle because it results in a net consumption of 4 ATP with no other overall effect. However, it allows many different types of extrahepatic cells to work at the expense of the liver.[6]
Triglyceride/fatty acid cycle
In the triglyceride/fatty acid cycle, triglycerides in adipose tissue are partially or completely hydrolyzed to free fatty acids and glycerol, in a process called lipolysis; the released fatty acids are then used to resynthesize new molecules of triglycerides.[3][6][7] Four moles of ATP are consumed for every mole of triglycerides that completes the cycle.
This futile cycle can take place:
- between adipose tissue, which releases fatty acids, and the liver, which re-esterified them to triglycerides, leading to a redistribution of stored energy;[4]
- in adipocytes, where it may contribute to thermogenesis and modifications of the stored fatty acids.
Regarding the modifications, the cycle renders fatty acids accessible for re-arrangements such as elongations and desaturations, which allow saturated fatty acids to be converted to unsaturated fatty acids. However, the efficiency of the process seem to depend on the type of fatty acids.[7] For example, the metabolism of the released medium-chain fatty acids is faster than the conversion of palmitic acid, one of the long-chain fatty acids, to palmitoleic acid, oleic acid, and then, in hepatocytes, to arachidonic acid.
Note that the conversion of medium-chain fatty acids and palmitic acid to long-chain unsaturated fatty acids reduces the health risk associated to their accumulation in stored triglycerides.[5]
Generation of heat
In some cases a futile cycle has the only function of producing heat through the hydrolysis of ATP. This occurs, for example, in the flight muscles of bumblebees, which, in order to fly, must maintain a thoracic temperature of about 30 °C, even when the external temperature is 10 °C. The thoracic temperature is maintained at the optimal levels for flight thanks to the futile cycle between the reactions catalyzed by PFK-1 and FBPase. In fact, flight muscle FBAase is not inhibited by AMP, which suggests that, during evolution, this protein has been selected for the generation of heat.
Unlike bumblebees, flight muscles of honeybees contain almost no FBPase, and therefore these insects cannot fly when the external temperature is low.[2]
References
- ^ Newsholme E.A. Substrate cycles: their metabolic, energetic and thermic consequences in man. Biochem Soc Symp. 1978;43:183–205.
- ^ a b c Berg J.M., Tymoczko J.L., and Stryer L. Biochemistry. 5th Edition. W. H. Freeman and Company, 2002
- ^ a b Reshef L., Olswang Y., Cassuto H., Blum B., Croniger C.M., Kalhan S.C., Tilghman S.M., Hanson R.W. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem 2003;278(33):30413-6. doi:10.1074/jbc.R300017200
- ^ a b Sharma A.K., Wolfrum C. Lipid cycling isn’t all futile. Nat Metab 2023;5(4):540-541. doi:10.1038/s42255-023-00779-x
- ^ a b Wunderling K., Zurkovic J., Zink F., Kuerschner L., Thiele C. Triglyceride cycling enables modification of stored fatty acids. Nat Metab 2023;5(4):699-709. doi:10.1038/s42255-023-00769-z
- ^ a b c Brownstein A.J., Veliova M., Acin-Perez R., Liesa M., Shirihai O.S. ATP-consuming futile cycles as energy dissipating mechanisms to counteract obesity. Rev Endocr Metab Disord 2022;23(1):121-131. doi:10.1007/s11154-021-09690-w
- ^ a b c Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 6th Edition. W.H. Freeman and Company, 2012
- ^ a b Garrett R.H., Grisham C.M. Biochemistry. 4th Edition. Brooks/Cole, Cengage Learning, 2010