Green tea, like the other types of tea, is an infusion of dried and processed leaves of Camellia sinensis, a member of the Theaceae family.
The processing of the leaves leading to the product ready for use is such as to minimize the oxidation of the compounds contained in them, particularly phytochemicals such as catechins, which are polyphenols belonging to the class of flavonoids and the most responsible for the health benefits of green tea.
Having undergone no significant chemical modifications, leaves retain green color, whereas the beverage, prepared with one tea bag per person, or in case of loose tea, one teaspoon per person, for an infusion time of about 3 minutes in water at 75 °C, is golden yellow in color.
Some organoleptic properties of green tea, such as the flavor, that is more delicate and lighter than that of black tea, and the health properties, which have always been recognized in East Asia cultures, depend on leaf processing.
Only recently scientists started studying the health benefits of tea consumption, highlighting its role in preventing many diseases, such as cardiovascular diseases and some types of cancer.
It has been shown that tea polyphenols, particularly catechins, are able to activate intracellular signaling pathways by binding to membrane receptors and/or entering the cell and binding to cytoplasmic, mitochondrial or nuclear receptors. Then, depending on the cell type, they activate or inhibit some cellular processes.
Given the high consumption of tea in the world, even small effects on health could have significant effects on public health.
Contents
Camellia sinensis
Camellia sinensis is an evergreen plant native to South, East, and Southeast Asia, which is now cultivated in at least 30 countries, mostly in tropical or subtropical climates, although some varieties grow in Cornwall and Washington State.
In nature, Camellia sinensis can grow up to 15-20 meters (49-65 ft), whereas in plantations it is pruned to less than 1,5 meters to facilitate leaf harvesting.
It can grow up to altitude of 1,500-2,000 meters (4,900-6,550 ft), and many of the high-quality teas are produced from such crops, as the plant grows slowly and the leaves acquire a better flavor.
The most cultivated varieties, of the four known, are Camellia sinensis var. sinensis, native to China, and Camellia sinensis var. assamica, native to India.
The different types of tea are produced from fresh leaves. Young leaves are preferred over older leaves that are considered to be inferior in quality.
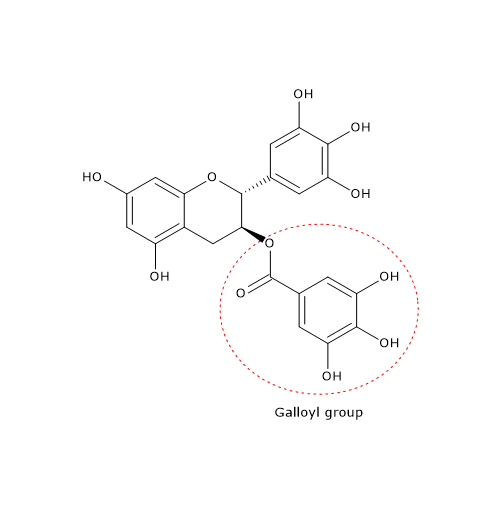
Fresh leaves are rich in water-soluble polyphenols, especially catechins and glycosylated catechins. The major catechins in green tea are epigallocatechin-3-gallate or EGCG, the most active, epigallocatechin, epicatechin 3-gallate, epicatechin. Catechin, gallocatechin, catechin gallate, and gallocatechin gallate are also present, although in lower amount.
These polyphenols account for 30-42 percent of the dry leaf weight. Caffeine accounts for 1,5-4,5 percent of the dry leaf weight.
In addition to leaf processing, the organoleptic properties of the beverage are influenced by cultivar, characteristics of the soil where the plant grown up, methods of cultivation, altitude, climate, and time of year in which leaf harvest occurs.
How green tea is made
The differences in leaf processing, which lead to the different types of tea ready for consumption, cause different degrees of oxidation of the compounds present in them, especially catechins.
During green tea manufacturing, oxidative processes, both enzymatic and chemical, are minimized.
After harvesting, leaves are exposed to sunlight for 2-3 hours and withered/dried. Then, the processing proceeds through three steps:
- heat treatment;
- rolling;
- drying.
Heat treatment, short and gentle, is crucial for the quality and properties of the beverage. It can done either with a steam, the traditional Japanese method, or by dry cooking in hot pans, that is similar to a roasting method and is the traditional Chinese method. Heat treatment inactivates enzymes and then prevents the enzymatic oxidation processes, particularly those involving polyphenols. It also removes the grassy smell, and evaporates, in the case of the traditional Chinese method, part of the water of the leaf, which constitutes about 75 percent of its weight, making it softer, thus facilitating the next step.
Heat treatment is followed by the rolling step, that facilitates the subsequent drying step and, destroying the leaf tissue, favors the release of aromas, thus improving the quality of the product.
The drying, the last step, improves the appearance of beverage and leads to the production of new compounds.
Health benefits
In East Asia cultures, mainly in China and Japan, tea drinking has always been associated with health benefits. Below is a brief review of the results of epidemiological and laboratory studies that have analyzed the effects that green tea consumption can play in preventing many diseases. EGCG, which is the most abundant catechin in green tea accounting for about 60 percent of the polyphenols present in dried leaves, seems to play the main role.
At the molecular level, the galloyl groups at positions 3 and/or 3′ appear to be essential for many of the effects exerted by catechins.
Cardiovascular disease
Cardiovascular disease is the main cause of deaths worldwide, particularly in low- and middle-income countries, with an estimate of about 17 million deaths in 2008 that could increase up to 23.3 million by 2030.
Daily tea consumption, especially green tea, seems to be associated with a reduced risk of developing cardiovascular disease, such as hypertension and stroke.
Among the proposed mechanisms, the improved bioactivity of the endothelium-derived vasodilator nitric oxide, due to the action of tea polyphenols that could enhance nitric oxide synthesis and/or decrease its breakdown by superoxide anions, seem to be important.
Cancer
Several epidemiological and laboratory studies have shown encouraging results with respect to the preventive role of tea consumption, especially green tea, against the development of some cancers such as those of the oral cavity, digestive tract, and lung among those who have never smoked.
Tea polyphenols seem to act not only as antioxidants, but also as compounds that, directly, can influence gene expression and various metabolic pathways.
Antiviral activity
Recent studies have highlighted antiviral effects of catechins, particularly EGCG of green tea and theaflavins of black tea, especially against positive single-stranded RNA viruses, to which the Coronaviridae family, and then SARS-CoV-1 and SARS-CoV-2, belongs.
The antiviral properties of EGCG appear to be due to its structural characteristics, namely, the presence of pyrogallic and galloyl groups.
Starch digestion
In vitro studies have shown that green tea and black tea polyphenols can reduce the glycemic index of starchy foods. Hence, they could be useful in controlling their glycemic index in vivo. This effect seems to be due to the inhibition of pancreatic alpha-amylase and other digestive enzymes, and to a direct interaction between starch and phytochemicals that would reduce the surface area of starch granules available for enzyme activity. Green tea appears to be equally effective against both gluten-containing foods, against which black tea appears less effective, and gluten free foods.
Weight loss
During weight loss and weight-loss maintenance it is important to keep as constant as possible the daily energy expenditure.
Since the 90s, it has been proposed that green tea, by virtue of its content of caffeine and catechins could be of help for:
- maintaining, or even increasing, daily energy expenditure;
- increasing fat oxidation.
In addition to these potential lipolytic and thermogenic effects, catechins and caffeine could act on other targets, such as lipid absorption and energy intake, perhaps through their effect on gut microbiota, which is part of the larger human microbiota, and gene expression.
And products for weight loss and weight maintenance based on green tea extracts have been marketed. It should be noted that these products contain catechins and caffeine in much higher amount than beverage.
How much truth is there in green tea’s fat burning effect?
The issue seems to have been resolved by a meta-analysis of 15 studies on weight loss and intake of these products. The study showed that green tea-based products induces, in overweight and obese adults, a weight loss that is:
- not statistically significant;
- very small;
- probably not clinically important.
Hence, on the basis of scientific evidence, green tea does not seem to be helpful in fat loss nor in weight maintenance.
Anticariogenic activity
Animal and in vitro studies have shown that tea, and particularly its polyphenols, seem to possess:
- antibacterial activity against cariogenic bacteria, such as Streptococcus mutans, as in the case of green tea EGCG;
- inhibitory action on salivary and bacterial amylase, in which black tea thearubigins and theaflavins are more effective than green tea catechins;
- inhibitory action on acid production in the oral cavity.
All these properties make green tea and black tea beverages with potential anticariogenic activity.
References
- Arab L., Khan F., and Lam H. Tea consumption and cardiovascular disease risk. Am J Clin Nutr 2013;98:1651S-1659S. doi:10.3945/ajcn.113.059345
- Clifford M.N., van der Hooft J.J.J., and Crozier A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am J Clin Nutr 2013;98:1619S-1630S. doi:10.3945/ajcn.113.058958
- Dwyer J.T. and Peterson J. Tea and flavonoids: where we are, where to go next. Am J Clin Nutr 2013;98:1611S-1618S. doi:10.3945/ajcn.113.059584
- Goenka P., Sarawgi A., Karun V., Nigam A.G., Dutta S., Marwah N. Camellia sinensis (Tea): implications and role in preventing dental decay. Phcog Rev 2013;7:152-156. doi:10.4103/0973-7847.120515
- Green R.J., Murphy A.S., Schulz B., Watkins B.A. and Ferruzzi M.G. Common tea formulations modulate in vitro digestive recovery of green tea catechins. Mol Nutr Food Res 2007;51(9):1152-1162. doi:10.1002/mnfr.200700086
- Grassi D., Desideri G., Di Giosia P., De Feo M., Fellini E., Cheli P., Ferri L., and Ferri C. Tea, flavonoids, and cardiovascular health: endothelial protection. Am J Clin Nutr 2013;98:1660S-1666S. doi:10.3945/ajcn.113.058313
- Huang W-Y., Lin Y-R., Ho R-F., Liu H-Y., and Lin Y-S. Effects of water solutions on extracting green tea leaves. Sci World J 2013;Article ID 368350. doi:10.1155/2013/368350
- Hursel R. and Westerterp-Plantenga M.S. Catechin- and caffeine-rich teas for control of body weight in humans. Am J Clin Nutr 2013;98:1682S-1693S. doi:10.3945/ajcn.113.058396
- Hursel R., Viechtbauer W. and Westerterp-Plantenga M.S. The effects of green tea on weight loss and weight maintenance: a meta-analysis. Int J Obesity 2009;33:956-961. doi:10.1038/ijo.2009.135
- Jurgens T.M., Whelan A.M., Killian L., Doucette S., Kirk S., Foy E. Green tea for weight loss and weight maintenance in overweight or obese adults. Editorial group: Cochrane Metabolic and Endocrine Disorders Group. 2012:12 Art. No.: CD008650. doi:10.1002/14651858.CD008650.pub2
- Lambert J.D. Does tea prevent cancer? Evidence from laboratory and human intervention studies. Am J Clin Nutr 2013;98:1667S-1675S. doi:10.3945/ajcn.113.059352
- Lorenz M. Cellular targets for the beneficial actions of tea polyphenols. Am J Clin Nutr 2013;98:1642S-1650S. doi:10.3945/ajcn.113.058230
- Mathur A., Gopalakrishnan D., Mehta V., Rizwan S.A., Shetiya S.H., Bagwe S. Efficacy of green tea-based mouthwashes on dental plaque and gingival inflammation: a systematic review and meta-analysis. Indian J Dent Res 2018;29(2):225-232. doi:10.4103/ijdr.IJDR_493_17
- Mhatre S., Srivastava T., Naik S., Patravale V. Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: a review. Phytomedicine 2020;153286. doi:10.1016/j.phymed.2020.153286
- Sharma V.K., Bhattacharya A., Kumar A. and Sharma H.K. Health benefits of tea consumption. Trop J Pharm Res 2007;6(3):785-792.
- Yang Y-C., Lu F-H., Wu J-S., Wu C-H., Chang C-J. The protective effect of habitual tea consumption on hypertension. Arch Intern Med 2004;164:1534-1540. doi:10.1001/archinte.164.14.1534
- Yuan J-M. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr 2013;98:1676S-1681S. doi:10.3945/ajcn.113.058271
- Xu J., Xu Z., Zheng W. A review of the antiviral role of green tea catechins. Molecules 2017;22(8):1337. doi:10.3390/molecules22081337
