Maltose, also known as malt sugar or α-D-glucopyranosyl-(1→4)-D-glucopyranose, is a disaccharide composed of two D-glucose molecules in their pyranose form. The two monosaccharides are linked by an α-(1→4) glycosidic bond, a covalent bond between C1 of one glucose unit, its hemiacetal anomeric carbon, and the oxygen atom of the hydroxyl group at C4 of the second glucose unit. The bond forms with retention of configuration at C1, remaining in the α-configuration. Because one glucose residue retains a hemiacetal carbon, maltose is a reducing sugar.[1][2]
Maltose is produced in the proximal section of the small intestine, the duodenum, as well as in germinating seeds, through the action of amylases on starch.[3]
Industrially, it is obtained through acidic or enzymatic hydrolysis of starches of various origins, often using fungal or bacterial amylases, such as those from Aspergillus oryzae or Bacillus subtilis.[4][5]
Maltose does not naturally occur in foods; it is found only in certain processed foods to which it is added during manufacturing.[6] For example, it is present in many bakery and pastry products, where it functions as a sweetener, stabilizer, and preservative.[7] For this reason, it is included among authorized food additives.[8]
In the duodenum, the α-(1→4) glycosidic bond is hydrolyzed by brush-border membrane hydrolases of the enterocytes. The released glucose is then absorbed and enters the bloodstream.[9]
Contents
Chemical properties
As with the disaccharides lactose, sucrose, and trehalose, its molecular formula is C12H22O11 and its molecular weight is 342.30 g/mol.[10]
It is highly soluble in water and has a sweet taste, although it is only about 33% as sweet as sucrose.[11]
Like monosaccharides, and like lactose among disaccharides, it is a reducing sugar, since the α-(1→4) glycosidic bond does not involve the hemiacetal (anomeric) carbon of one of the two glucose residues. This carbon is therefore free to revert to the carbonyl form; in solution, the ring can open to expose a free aldehyde group. However, it should be noted that the open-chain aldehyde form is present only in very small amounts.[2]
The anomeric carbon not involved in the glycosidic bond undergoes mutarotation; that is, both the α and β configurations are possible, although the β configuration predominates.
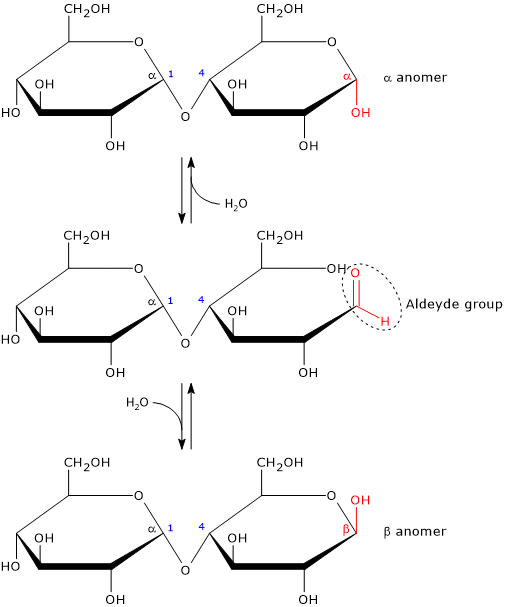
Anomerism is a form of optical isomerism characteristic of carbohydrates. Two stereoisomers of a cyclic monosaccharide are defined as anomers if they differ only in the configuration at the hemiacetal or hemiketal carbon, known as the anomeric carbon or anomeric center.[1]
Intramolecular cyclization of monosaccharides converts the carbonyl carbon into an asymmetric (chiral) center. In solution, and in equilibrium with the open-chain form, two anomers can form:
- α-isomer, if, during cyclization, the oxygen of the hydroxyl group attacks the carbon atom of the carbonyl group from top side of the sp2 plane;
- β-isomer, if the oxygen of the hydroxyl group attacks the carbon atom of the carbonyl group from bottom side of the sp2 plane.[12]
Role
During seed germination, endospermic starch is hydrolyzed by amylases to maltose and glucose, which, along with other hydrolysis products, support the growth of germinating seedlings.[13]
The production of alcoholic beverages obtained by cereal fermentation, as well as the manufacture of foods with a high maltose content, such as glucose and maltose syrups, or bread-making, also relies on amylase activity on starch to release the disaccharide.[3][14]
In pastry making, maltose is used both as a sweetener and as a stabilizer for icings, without increasing sweetness to the same extent as sucrose. Furthermore, because it can inhibit starch retrogradation it can extend shelf life.[7] For these reasons, maltose can be classified as a preservative, stabilizer, or sweetener for food use. It is also present in many carbohydrate preparations for infant feeding.[8]
Outside the food industry, it is used, for example, as a stabilizer for immunoglobulins.[10][15]
Maltose digestion
In humans, carbohydrate digestion begins in the oral cavity with salivary alpha-amylase and continues in the duodenum through the action of hydrolases such as pancreatic α-amylase and the brush-border enzymes of enterocytes. The combined activity of these enzymes enables the hydrolysis of disaccharides, oligosaccharides, and polysaccharides into their constituent monosaccharides: fructose, glucose, and galactose. The absorption of monosaccharides occurs in the small intestine and is mediated by specific protein transporters located in the plasma membrane of enterocytes.[9]
The action of salivary and pancreatic α-amylase on the two polysaccharides that compose starch, amylose and amylopectin, produces maltose, maltotriose, an oligosaccharide composed of three glucose units linked by α-(1→4) glycosidic bonds, and, from amylopectin, α-limit dextrins, which are glucose polymers containing at least one α-(1→6) glycosidic bond.[16][17]
Although maltose, maltotriose, and α-limit dextrins can also arise from glycogen breakdown, this source plays a negligible role because, after the animal’s death, glycogen undergoes rapid degradation, mainly to glucose and lactic acid.[18]
The α-(1→4) glycosidic bond of maltose is hydrolyzed in a reaction catalyzed by two enzymes: sucrase-isomaltase (EC 3.2.1.48 and 3.2.1.10) and maltase-glucoamylase, or MAG (EC 3.2.1.20 and 3.2.1.3).[2]
Sucrase-isomaltase
Sucrase-isomaltase is a bifunctional enzyme with two active sites.[19][20] One active site, sucrase, is an α-glucosidase that hydrolyzes the glycosidic bonds of maltose, sucrose, and short α-(1→4) linked glucose oligomers containing up to six glucose units.[21] Sucrase accounts for 60–80% of small-intestine maltase activity.[22] The other active site, isomaltase, is an α-(1→6) glycosidase that hydrolyzes the α-(1→6) glycosidic bonds in α-limit dextrins.[23]
Maltase-glucoamylase
Like sucrase-isomaltase, maltase-glucoamylase is an enzyme with two active sites. One active site has high specificity for maltose, whereas the other has broad substrate specificity and acts on glucose oligomers. Both active sites catalyze the release of glucose units.[24][25][26]
Notably, α-amylase, sucrase-isomaltase, and maltase-glucoamylase act synergistically to completely digest dietary starches into glucose.[9][17]
Sucrase-isomaltase and MAG deficiency
Deficiency of a single brush-border glycosidase in enterocytes is generally due to a genetic defect, whereas the loss of all glycosidases is often the result of an intestinal infection. After the infection resolves, these enzymes gradually recover.[27]
Both sucrase and isomaltase activities may be impaired in congenital or primary sucrase-isomaltase deficiency, with accumulation, in the former case, also of undigested maltose.[28] A similar situation can occur in congenital maltase-glucoamylase deficiency, a rare condition with only a few cases reported in the literature.[29]
In both conditions, as well as during intestinal infections, undigested carbohydrates remain in the intestinal lumen, where they can be partially fermented by the gut microbiota, a component of the human microbiota. This fermentation leads to excessive production of gases such as hydrogen, carbon dioxide, and methane, and short-chain fatty acids, primarily acetic, propionic, and butyric acid.[30][31] The presence of undigested carbohydrates and their fermentation products, many of which are osmotically active solutes, causes an increase in intraluminal osmotic pressure, an influx of water into the lumen, and, consequently, diarrhea.[32]
Treatment consists of reducing or avoiding dietary maltose.[33]
References
- ^ a b Soderberg T. Organic chemistry with a biological emphasis. Volume II. Chemistry Publications. 2019. https://digitalcommons.morris.umn.edu/chem_facpubs/2
- ^ a b c Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 8th Edition. W.H. Freeman and Company, 2021.
- ^ a b Garrett R.H., Grisham C.M. Biochemistry. 6th Edition. Brooks/Cole, Cengage Learning, 2016.
- ^ Benjamin S., Smitha R.B., Jisha V.N., Pradeep S., Sajith S., Sreedevi S., Priji P., Unni K.N., Sarath Josh M.K. A monograph on amylases from Bacillus spp. Adv biosci biotechnol 2013;4(2):227-241. doi:10.4236/abb.2013.42032
- ^ Gopinath S.C., Anbu P., Arshad M.K., Lakshmipriya T., Voon C.H., Hashim U., Chinni S.V. Biotechnological processes in microbial amylase production. Biomed Res Int 2017;2017:1272193. doi:10.1155/2017/1272193
- ^ Crow R.R., Kumar S., Varela M.F. Maltose chemistry and biochemistry. In: Preedy V.R. editor. Dietary sugars: chemistry, analysis, function and effects. Cambridge: Royal Society of Chemistry; 2012. p. 101-114. doi:10.1039/9781849734929-00101
- ^ a b Wang S., Li C., Copeland L., Niu Q., Wang S. Starch Retrogradation: a comprehensive review. Compr Rev Food Sci Food Saf 2015;14:568-585. doi:10.1111/1541-4337.12143
- ^ a b EFSA. Food additives. Last reviewed date: 13 November 2025.
- ^ a b c Stipanuk M.H., Caudill M.A. Biochemical, physiological, and molecular aspects of human nutrition. 4th Edition. St. Louis: Elsevier, 2018.
- ^ a b National Center for Biotechnology Information. PubChem Compound Summary for CID 439186, Maltose. https://pubchem.ncbi.nlm.nih.gov/compound/Maltose. Accessed Nov. 19, 2025.
- ^ Spillane W.J. Optimising sweet taste in foods. Woodhead Publishing; 2006. p. 271.
- ^ Solomons T.W.G., Fryhle C.B., Snyder S.A. Solomons’ organic chemistry. 12th Edition. John Wiley & Sons Incorporated, 2017.
- ^ Andriotis V.M., Saalbach G., Waugh R., Field R.A., Smith A.M. The maltase involved in starch metabolism in barley endosperm is encoded by a single gene. PLoS One 2016;11(3):e0151642. doi:10.1371/journal.pone.0151642
- ^ Cauvain S.P, Young L.S. Technology of breadmaking. 2nd Edition. New York: Springer; 2007.
- ^ Alder L.B., Morgan L.A., Spickett G.P. Contribution of stabilizing agents present in intravenous immunoglobulin preparations to modulation of mononuclear cell proliferation in vitro. Scand J Immunol 1996;44(6):585-91. doi:10.1046/j.1365-3083.1996.d01-350.x
- ^ Butterworth P.J., Warren F.J. and Ellis P.R. Human α-amylase and starch digestion: an interesting marriage. Starch/Stärke 2011;63:395-405. doi:10.1002/star.201000150
- ^ a b Gangoiti J., Corwin S.F., Lamothe L.M., Vafiadi C., Hamaker B.R., Dijkhuizen L. Synthesis of novel α-glucans with potential health benefits through controlled glucose release in the human gastrointestinal tract. Crit Rev Food Sci Nutr 2020;60(1):123-146. doi:10.1080/10408398.2018.1516621
- ^ Terlouw E.M.C., Picard B., Deiss V., Berri C., Hocquette J.F., Lebret B., Lefèvre F., Hamill R., Gagaoua M. Understanding the determination of meat quality using biochemical characteristics of the muscle: stress at slaughter and other missing keys. Foods 2021;10(1):84. doi:10.3390/foods10010084
- ^ Hauri H.P., Quaroni A., Isselbacher K.J. Biogenesis of intestinal plasma membrane: posttranslational route and cleavage of sucrase-isomaltase. Proc Natl Acad Sci U S A 1979;76(10):5183-6. doi:10.1073/pnas.76.10.5183
- ^ Hunziker W., Spiess M., Semenza G., Lodish H.F. The sucrase-isomaltase complex: primary structure, membrane-orientation, and evolution of a stalked, intrinsic brush border protein. Cell 1986;46(2):227-34. doi:10.1016/0092-8674(86)90739-7
- ^ Quezada-Calvillo R., Sim L., Ao Z., Hamaker B.R., Quaroni A., Brayer G.D., Sterchi E.E., Robayo-Torres C.C., Rose D.R., Nichols B.L. Luminal starch substrate “brake” on maltase-glucoamylase activity is located within the glucoamylase subunit. J Nutr 2008;138(4):685-92. doi:10.1093/jn/138.4.685
- ^ Gericke B., Schecker N., Amiri M., Naim H.Y. Structure-function analysis of human sucrase-isomaltase identifies key residues required for catalytic activity. J Biol Chem 2017;292(26):11070-11078. doi:10.1074/jbc.M117.791939
- ^ Quezada-Calvillo R., Robayo-Torres C.C., Ao Z., Hamaker B.R., Quaroni A., Brayer G.D., Sterchi E.E., Baker S.S., Nichols B.L. Luminal substrate “brake” on mucosal maltase-glucoamylase activity regulates total rate of starch digestion to glucose. J Pediatr Gastroenterol Nutr 2007 Jul;45(1):32-43. doi:10.1097/MPG.0b013e31804216fc
- ^ Nichols B.L., Eldering J., Avery S., Hahn D., Quaroni A., Sterchi E. Human small intestinal maltase-glucoamylase cDNA cloning. Homology to sucrase-isomaltase. J Biol Chem 1998;273(5):3076-81. doi:10.1074/jbc.273.5.3076
- ^ Sim L., Quezada-Calvillo R., Sterchi E.E., Nichols B.L., Rose D.R. Human intestinal maltase-glucoamylase: crystal structure of the N-terminal catalytic subunit and basis of inhibition and substrate specificity. J Mol Biol 2008;375(3):782-92. doi:10.1016/j.jmb.2007.10.069
- ^ Rose D.R., Chaudet M.M., Jones K. Structural studies of the Intestinal α-glucosidases, maltase-glucoamylase and sucrase-isomaltase. J Pediatr Gastroenterol Nutr 2018;66 Suppl 3:S11-S13. doi:10.1097/MPG.0000000000001953
- ^ Viswanathan L., Rao S.S. Intestinal disaccharidase deficiency in adults: evaluation and treatment. Curr Gastroenterol Rep 2023;25(6):134-139. doi:10.1007/s11894-023-00870-z
- ^ Senftleber N.K., Ramne S., Moltke I., Jørgensen M.E., Albrechtsen A., Hansen T., Andersen M.K. Genetic loss of sucrase-isomaltase function: mechanisms, implications, and future perspectives. Appl Clin Genet 2023;16:31-39. doi:10.2147/TACG.S401712
- ^ Nichols B.L., Avery S.E., Karnsakul W., Jahoor F., Sen P., Swallow D.M., Luginbuehl U., Hahn D., Sterchi E.E. Congenital maltase-glucoamylase deficiency associated with lactase and sucrase deficiencies. J Pediatr Gastroenterol Nutr 2002;35(4):573-9. doi:10.1002/j.1536-4801.2002.tb07889.x
- ^ Holtug K., Clausen M.R., Hove H., Christiansen J., Mortensen P.B. The colon in carbohydrate malabsorption: short-chain fatty acids, pH, and osmotic diarrhoea. Scand J Gastroenterol 1992;27(7):545-52. doi:10.3109/00365529209000118
- ^ Oliphant K., Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 2019;7(1):91. doi:10.1186/s40168-019-0704-8
- ^ Weijers H.A., va de Kamer J.H., Mossel D.A., Dicke W.K. Diarrhoea caused by deficiency of sugar-splitting enzymes. Lancet. 1960;2(7145):296-7. doi:10.1016/s0140-6736(60)91381-7
- ^ Gericke B., Amiri M., Naim H.Y. The multiple roles of sucrase-isomaltase in the intestinal physiology. Mol Cell Pediatr 2016;3(1):2. doi:10.1186/s40348-016-0033-y