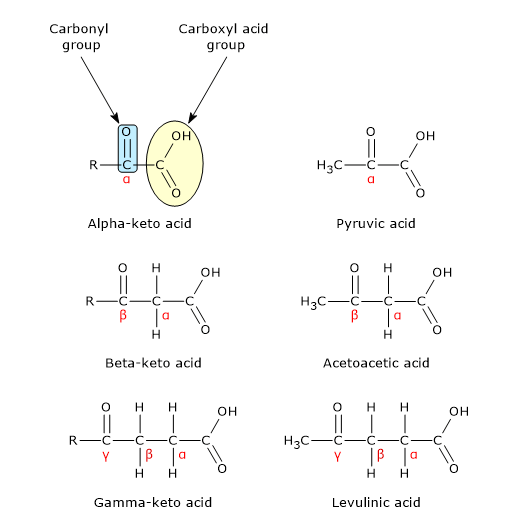
Keto acids or ketoacids are organic compounds containing two functional groups: a carboxyl acid group (−COOH) and a carbonyl group (˂C=O).
Based on the position of the carbonyl group relative to the carboxylic acid group, to which the IUPAC nomenclature rules assign the highest priority, ketoacids are classified as alpha-keto acids, beta-keto acids, and gamma-keto acids.[2]
 Keto acids, and in particular alpha-keto acids, are very important in biochemistry, being involved in many metabolic pathways.[4]
Keto acids, and in particular alpha-keto acids, are very important in biochemistry, being involved in many metabolic pathways.[4]
Alpha-keto acids
They have the carbonyl group at the first carbon from the carboxylic acid. Many of these compounds, in the form of their conjugate bases, have important biological functions. Below are some examples.
Pyruvic acid, the simplest alpha-keto acid, is the end metabolic product of glycolysis.
Oxaloacetic acid and alpha-ketoglutaric acid are intermediates of the citric acid cycle.
Alpha-keto acids can arise from transamination and oxidative deamination reactions of amino acids. In transamination reactions, the alpha amino group of the amino acid is transferred to an alpha-keto acid, usually alpha-ketoglutarate, with the formation of a new amino acid and an new alpha-keto acid. These reactions are catalyzed by enzymes called aminotransferases or transaminases (EC6.1.-).
alpha-Keto acid + Amino acid ⇄ New amino acid + New alpha-keto acid
In oxidative deaminations, amino acids are converted into the corresponding alpha-keto acids by removing the amino group, which is converted to ammonia and replaced by a carbonyl group. Since the reaction is reversible, ketoacids are also precursors of amino acids.
Note: ammonia is a toxic compound, and is converted into the safer compound urea via the urea cycle in the liver.[4]
Pyruvate, oxaloacetate and alpha-ketoglutarate, the latter via oxaloacetate, are the entry points into gluconeogenesis of the carbon skeleton of many glucogenic amino acids.[4]
It has also been observed that, in vitro, murine and human tumor cell lines secrete 2-chetoacids into the tumor microenvironment, such as α-ketoisocaproate, α-keto-β-methylvalerate and α-ketoisovalerate, which are capable to influence the anti-tumor activity of macrophages.[1]
Beta-keto acids
They have the carbonyl group at the second carbon from the carboxylic acid.
Examples of beta-keto acids are acetoacetic acid, the simplest one, and beta-hydroxybutyric acid, which are two of the three ketone bodies, together with acetone, produced by the hepatocyte when acetyl-CoA is produced in excess of the capacity of citrate synthase (EC 2.3.3.1), namely, of citric acid cycle to oxidize it fully, as during prolonged fasting or diets very low in carbohydrates.
Note that acetoacetyl-CoA and beta-hydroxybutyryl-CoA, namely, the activated forms of these beta-keto acids, are also intermediates in the butyric acid synthesis pathway which occurs in most butyrate-producing bacteria of the gut microbiota.[3][5][6]
Gamma-keto acids
They have the carbonyl group at the third carbon from the carboxylic acid.
An example is levulinic acid, the simplest one, which arises from the catabolism of cellulose.
References
- ^ Cai Z., Li W., Brenner M., Bahiraii S., Heiss E.H., Weckwerth W. Branched-chain ketoacids derived from cancer cells modulate macrophage polarization and metabolic reprogramming. Front Immunol 2022;13:966158. doi:10.3389/fimmu.2022.966158
- ^ IUPAC, Pure Appl Chem 2020. doi:10.1515/pac-2019-0104
- ^ Miller T.L., Wolin M.J. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl Environ Microbiol 1996;62(5):1589-92. doi:10.1128/aem.62.5.1589-1592
- ^ a b c Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 6th Edition. W.H. Freeman and Company, 2012
- ^ Portincasa P., Bonfrate L.,Vacca M., De Angelis M., Farella I., Lanza E., Khalil M.,Wang D.Q.-H., Sperandio M., Di Ciaula A. Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int J Mol Sci 2022;23:1105. doi:10.3390/ijms23031105
- ^ Pryde S.E., Duncan S.H., Hold G.L., Stewart C.S., Flint H.J. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett 2002;217(2):133-9. doi:10.1111/j.1574-6968.2002.tb11467.x