The leaves of the tea plant, Camellia sinensis, are rich in compounds with many biological activities, ranging from preventing the development of chronic diseases to reducing the glycemic index of starchy foods.
More than 4000 different molecules have been found in the beverage, of which about one third of these are polyphenols, the most important phytochemicals in determining the nutritional values and health benefits of the tea.
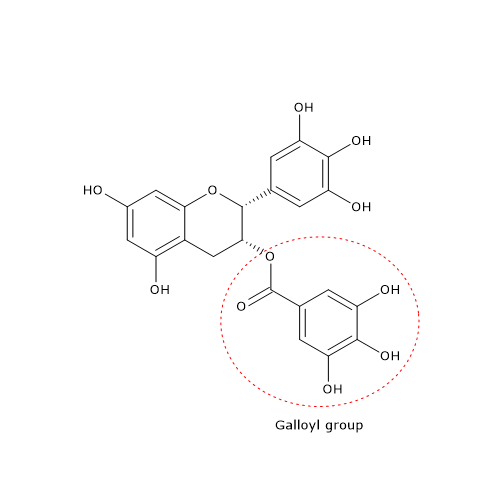
Tea polyphenols are mostly flavonoids. Examples are catechins in green tea, among which epigallocatechin-3-gallate or EGCG is the most important and abundant, and thearubigins and theaflavins in black tea. Their galloyl groups at positions 3 and/or 3′ appear to be particularly important for their effects.
Other bioactive compounds present in tea leaves are:
- alkaloids, such as caffeine, theophylline and theobromine;
- amino acids, and among them, theanine or R-glutamylethylamide, that is also a brain neurotransmitter and one of the most important amino acids in green tea;
- proteins;
- carbohydrates;
- chlorophyll;
- volatile organic molecules, that contribute to the aroma of the beverage;
- fluoride, aluminum and trace elements.
Contents
Biological activities
Polyphenols, both in vivo and in vitro, have a broad spectrum of biological activities, such as:
- antioxidant and prooxidant properties;
- a protective role against the development of diabetes, hyperlipidemia, and various types of tumors;
- inhibition of inflammation;
- antiviral activities;
- anticariogenic activity.
Hence, there has been a growing interest in recent years toward the possible preventive effects of tea against many diseases, particularly cardiovascular disease, for example in the development and progression of atherosclerosis.
Mechanisms of action
Currently, knowledge is accumulating on the effects of tea polyphenols at cellular and molecular level.
It seems, at least in vitro, that catechins, and theaflavins and thearubigins are the compounds responsible for the physiological effects and health benefits of green tea and black tea, respectively.
Among the molecular mechanisms by which tea polyphenols seem to exert their effects, it has been observed, after binding to specific cell membrane receptors, a change in the activity of various protein kinases that then phosphorylate target proteins, such as transcription factors, that, in turn, translocate into the nucleus and modify the gene expression. This appears to be the mechanism of action of EGCG, and the mechanism proposed for thearubigins, polymeric polyphenols that, due to their large dimensions, may not be able to cross the plasma membrane.
In addition, some polyphenols could be able to cross the plasma membrane, then binding to specific cytoplasmic, mitochondrial or nuclear targets.
And, depending on the cell type and their amount, tea polyphenols can activate or inhibit certain cellular processes.
Starch digestion
Tea polyphenols exert an inhibitory effect on starch digestion.
In vitro studies have shown that green tea extracts, which contain monomeric polyphenols, have an equal inhibitory effect on starch digestibility of wheat bread and gluten free bread, whereas black tea extracts, rich in tannins, namely, polymeric polyphenols, are less effective against wheat bread. Therefore, it seems that the inhibitory effect of tannins is negatively influenced by gluten, whereas gluten has a lower inhibitory effect on monomeric polyphenols.
The inhibitory effect of these phytochemicals has been attributed to various molecular mechanisms briefly described below.
- A competitive inhibition on pancreatic alpha-amylase. The galloyl groups are thought to be important for this effect.
- The inhibition of other digestive enzymes present in the gastrointestinal tract.
- The direct interaction with starch. Tea polyphenols can interact with starch granules through hydrogen bonds and hydrophobic forces, thus reducing the available surface to react with digestive enzymes.
- Conversely, gluten could reduce the amount of polyphenols able to interact with starch and therefore able to inhibit its digestion.
Tea polyphenols could represent a means for controlling the glycemic index of starchy foods. However, it should be emphasized that, for example in the case of bread, to achieve an inhibitory effect, 100 g of bread must be co-digested with 2.5 cups of green tea or 2 cups of black tea.
References
- Arab L., Khan F., and Lam H. Tea consumption and cardiovascular disease risk. Am J Clin Nutr 2013;98:1651S-1659S. doi:10.3945/ajcn.113.059345
- Dwyer J.T. and Peterson J. Tea and flavonoids: where we are, where to go next. Am J Clin Nutr 2013;98:1611S-1618S. doi:10.3945/ajcn.113.059584
- Grassi D., Desideri G., Di Giosia P., De Feo M., Fellini E., Cheli P., Ferri L., and Ferri C. Tea, flavonoids, and cardiovascular health: endothelial protection. Am J Clin Nutr 2013;98:1660S-1666S. doi:10.3945/ajcn.113.058313
- Kan L., Capuano E., Fogliano V., Oliviero T. and Verkerk R. Tea polyphenols as a strategy to control starch digestion in bread: the effects of polyphenol type and gluten. Food Funct 2020;11:5933-5943. doi: 10.1039/D0FO01145B
- Lambert J.D. Does tea prevent cancer? Evidence from laboratory and human intervention studies. Am J Clin Nutr 2013;98:1667S-1675S. doi:10.3945/ajcn.113.059352
- Lenore Arab L., Khan F., and Lam H. Tea consumption and cardiovascular disease risk. Am J Clin Nutr 2013;98:1651S-1659S. doi:10.3945/ajcn.113.059345
- Lorenz M. Cellular targets for the beneficial actions of tea polyphenols. Am J Clin Nutr 2013;98:1642S-1650S. doi:10.3945/ajcn.113.058230
- Mathur A., Gopalakrishnan D., Mehta V., Rizwan S.A., Shetiya S.H., Bagwe S. Efficacy of green tea-based mouthwashes on dental plaque and gingival inflammation: a systematic review and meta-analysis. Indian J Dent Res 2018;29(2):225-232. doi:10.4103/ijdr.IJDR_493_17
- Mhatre S., Srivastava T., Naik S., Patravale V. Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: a review. Phytomedicine 2020;153286. doi:10.1016/j.phymed.2020.153286
- Sharma V.K., Bhattacharya A., Kumar A. and Sharma H.K. Health benefits of tea consumption. Trop J Pharm Res 2007;6(3):785-792.
- Yuan J-M. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr 2013;98:1676S-1681S. doi:10.3945/ajcn.113.058271
- Xu J., Xu Z., Zheng W. A review of the antiviral role of green tea catechins. Molecules 2017;22(8):1337. doi:10.3390/molecules22081337