Galactose is a six-carbon monosaccharide.
Isolated for the first time by Louis Pasteur in 1855 from milk, its structure was clarified thirty years later by Kiliani H. and Fischer E.[1] In 1860, Berthelot P.E.M. referred to it as lactic glucose or galactose, from the Greek galaktos, which means milk, followed by the suffix for sugars, –ose.[2]
It occurs in both open-chain and cyclic forms. The open chain form has a carbonyl group at one end of the chain, which makes it an aldehyde derivative, therefore an aldohexose and a reducing sugar. In the cyclic form, it has four anomeric stereoisomers.[3]
The main dietary source is the disaccharide lactose, which is made up of a galactose molecule and a glucose molecule joined by a β-(1→4) glycosidic bond. In the small intestine, the glycosidic bond is hydrolyzed in a reaction catalyzed by lactase (EC 3.2.1.108), producing galactose and glucose, which are then absorbed.[4]
It performs a wide range of functions. After initial conversion to UDP-galactose (which is then converted to UDP-glucose), through the Leloir pathway, it can be used for both anabolic and catabolic purposes, depending on the type of tissue and the energy state of the cell.[5][6]
A defect in one of the enzymes of the Leloir pathway leads to the abnormal accumulation of galactose-related chemicals, such as galactitol and galactonate, in many tissues and organs, and causes galactosemia.[7]
Contents
- Chemical properties
- Food sources
- Intestinal absorption
- Metabolism of galactose
- Galactosemia
- References
Chemical properties
Like all hexoses, such as glucose and fructose, galactose has the molecular formula C6H12O6 and a molecular weight of 180.156 g/mol.
It is about 33% as sweet as sucrose, and about half as sweet as glucose.[8]
Like all aldohexoses, galactose has two enantiomers that, based on Fischer-Rosanoff convention or D-L system, are called D-galactose and L-galactose. The latter is not naturally occurring in higher organisms, as it cannot be further metabolized. Therefore, the term galactose will be used to indicate D-galactose.
It can occur in linear (open-chain) or cyclic form. The open chain form, which may be represented by a Fischer projection, has an aldehyde group, hence, a carbonyl group, at one end of the chain.
The carbonyl group makes it a reducing sugar, like the other monosaccharides and the disaccharides lactose and maltose.[3]
Structure
The open-chain form is thermodynamically unstable and is present in trace amounts in solution.
Aldehydes can react with alcohols, so the linear form of galactose can cyclize via nucleophilic attack by the oxygen atom of one of the hydroxyl groups on the carbon atom of the carbonyl group, forming an intramolecular hemiacetal.[9]
Cyclic compounds with rings consisting of three or four atoms are much less stable than those consisting of five and, above all, six atoms, due to the high steric interactions, just like the cyclic alkanes cyclopropane and cyclobutane, which are less stable than cyclopentane and cyclohexane.
In its cyclic form, galactose exists as four stereoisomers. Two have a five-membered ring consisting of one oxygen atom and four carbon atoms, that is, a furanose ring, and two have a six-membered ring consisting of one oxygen atom and five carbon atoms, a pyranose ring.[10]
In solution, pyranose and furanose forms are in equilibrium with the open chain form but, since the furanose form is less stable than the pyranose form, the equilibrium is strongly shifted towards the latter.[3]
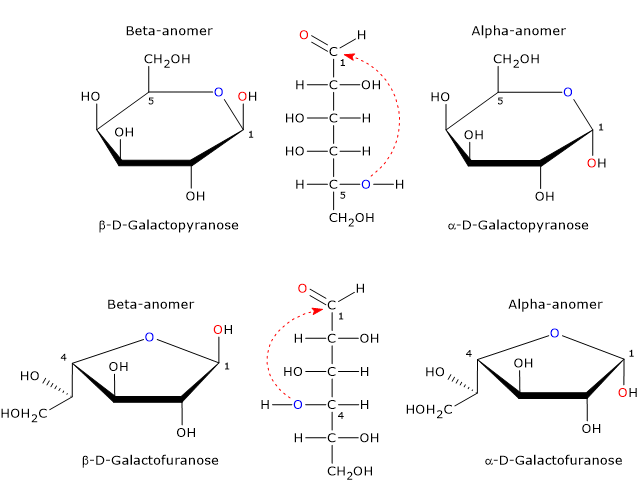
In mammals, the only cyclic form found is the pyranose form, which is an epimer of glucose, as it differs only by the configuration of the hydroxyl group on carbon 4. The furanose form is found in many lower organisms, both prokaryotes and eukaryotes, such as bacteria, green algae, fungi, protozoa (e.g. Trypanosomatids), sponges, and starfish. The furanose form is also found in pathogenic species, where it may play an important role, for example, in survival, while its biosynthetic pathway may be a target for the development of new drugs to treat infections caused by these pathogens.[10]
Anomers
The hemiacetal carbon, a tetrahedral atom that bears four different ligands, is a center of chirality.
Since nucleophilic attack by the oxygen atom can occur from top side or bottom site of the sp2 plane of the carbonyl carbon,
ring-closing reaction may yield two pyranose and two furanose configurations, which differ in the configuration of the hemiacetal carbon. The spontaneous interconversion between these forms in solution is called mutarotation, which yields two pairs of anomers.
Such epimers differ only in the configuration of the hemiacetal carbon and are called the α-anomer and β-anomer, while the hemiacetal carbon becomes the anomeric carbon or anomeric center.
The α-anomer is formed via nucleophilic attack from the top side of the sp2 plane, whereas β-anomer via nucleophilic attack from the bottom side of the sp2 plane.
Therefore, galactose can occur in five different forms: the open chain form plus four anomeric cyclic forms, namely, α-D-galactopyranose and β-D-galactopyranose, and α-D-galactofuranose and β-D-galactofuranose.[9]
Note: anomerism is a type of optical isomerism characteristic of monosaccharides.
Food sources
The main dietary sources are milk and dairy products, where it is mainly found as a component of lactose and, to a less extent, in more complex carbohydrates such as oligosaccharides and polysaccharides, called glycans, which are components of milk glycoproteins and glycolipids.[11]
It is also found in many other foods, such as fruit, vegetables, cereals, legumes, nuts, and honey, where it can occur in free form, bound to polyphenols, such as flavonols and anthocyanins, or as a component of glycoproteins and glycolipids.[12]
In the bound form, it is often linked by chemical bonds resistant to digestion.[13]
In the free form, it occurs in milligram amounts; below some examples:
- less than 0.1 mg/100 grams of edible portion: artichokes, mushrooms, olives, and peanuts;
- more than 10 mg/100 grams of edible portion: peppers, date, papaya, watermelon, tomato;
- up to 35.4 mg/100 grams of edible portion in persimmon.
Although these values are very low, they must be taken into account in case of galactosemia.[14]
Intestinal absorption
In mammals, carbohydrate digestion begins in the mouth and ends in the duodenum and jejunum, where hydrolases, such as pancreatic alpha-amylase and the disaccharidases of the brush border of enterocytes, hydrolyze disaccharides, oligosaccharides, and polysaccharides into their constituent monosaccharides: glucose, fructose, and galactose.[4]
The hydrolysis of lactose to produce D-glucose and β-D-galactose is catalyzed by the disaccharidase lactase, or lactase phlorizin-hydrolase. This enzyme has two active sites: one catalyzes the hydrolysis of the β-(1→4) glycosidic bond, and is therefore a beta-glucosidase; the other catalyzes the hydrolysis of phlorizin and glycolipids, such as ceramides, to yield fatty acids and sphingosine.[15]
Bacterial β-galactosidase (EC 3.2.1.23) can hydrolyze lactose into the two monosaccharides, too.[16]
The absorption of monosaccharides glucose and galactose takes place in the small intestine via the sodium-glucose linked transporter type I (SGLT1), an integral membrane protein that mediates their active transport across the brush border membrane of enterocytes. The transmembrane electrochemical gradient of sodium ions serves as the energy source for this transport system.[17]
Galactose leaves the enterocyte through the basolateral membrane by facilitated diffusion, mediated by the glucose transporter type 2 (GLUT2), enters the blood circulation, and is carried via the portal system to the liver, which absorbs approximately 90% of the circulating monosaccharide. Uptake into hepatocytes is also mediated by GLUT2.[17]
Galactose not absorbed by the liver can reach other organs, such as the lactating mammary gland.
Under physiological conditions, blood galactose concentration may increase following alcohol intake, which reduces both its intestinal absorption and hepatic metabolism.
Metabolism of galactose
In the absence of lactose or other dietary sources of galactose, the monosaccharide can be synthesized from glucose, as UDP-galactose 4-epimerase (EC 5.1.3.2), one of the four enzymes of the Leloir pathway, catalyzes a reversible reaction.[4]
In addition to the de novo synthesis and dietary intake, galactose deriving from the turnover of glycoproteins and glycolipids is also very important.[18]
Galactose may enter three main metabolic pathways, the most important of which is the Leloir pathway, by which it may be converted to glucose.
The other two pathways, an oxidative one, which leads to the formation of galactonate, and a reductive one, which leads to the conversion of galactose into galactitol, play marginal roles under physiological conditions. However, they may become substantially active in the presence of excessive levels of galactose, as in galactosemia.[6]
Leloir pathway
The Leloir pathway, discovered by Luis Federico Leloir and colleagues in 1948, leads through four enzymatic reactions to the inversion of the configuration of hydroxyl group at carbon 4 of galactose, ultimately yielding glucose.[5]
- β-galactose, a product of lactose digestion, is converted to the α-anomer in a reaction catalyzed by galactose mutarotase (EC 5.1.3.3).[19]
- In the next step, galactokinase (EC 2.7.1.6) catalyzes the phosphorylation of galactose to galactose 1-phosphate, which cannot diffuse out of the cell.[20] Furthermore, phosphorylation maintains a low intracellular concentration of the monosaccharide, thereby favoring its transport into the cell.
- Galactose 1-phosphate is activated to UDP-galactose in a reaction catalyzed by galactose 1-phosphate uridylyltransferase (EC 2.7.7.12).
- Finally, UDP-galactose is epimerized to UDP-glucose in a reaction catalyzed by UDP-galactose 4-epimerase.[20]
The Leloir pathway enables the activation of galactose to UDP-galactose, which may be used as sugar donor for glycosylation reactions of lipids and proteins.[6]
This allows galactose to play a key structural role in the early stages of development and to be a component of antigens present on erythrocytes, particularly those used to determine the AB0 blood group.
In lactating mammary gland, UDP-galactose is a precursor for lactose synthesis, accounting for about 30% of the galactose in lactose, and is also used in glycosylation of milk lipids and proteins.[4]
UDP-glucose, depending on the type of tissue and the energy state of the cell, may be:
- used in glycosylation reactions;
- directed into glycogen synthesis, which is the main metabolic fate of galactose in the liver;
- utilized in glycolysis for energy production;
- or funneled into gluconeogenesis, after conversion to glucose 1-phosphate in a reaction catalyzed by UDP-glucose pyrophosphorylase (EC 2.7.7.9), followed by isomerization to glucose 6-phosphate via phosphoglucomutase (EC 5.4.2.2).[6][18]
Since UDP-glucose pyrophosphorylase catalyzes a reversible reaction, the synthesis of UDP-galactose from galactose 1-phosphate and UTP may represent an alternative metabolic pathway in individuals with galactose-1-phosphate uridylyltransferase deficiency.[21]
Galactitol
An alternative metabolic pathway to the Leloir pathway leads to the reduction of galactose to galactitol, in a reaction catalyzed by aldose reductase (EC 1.1.1.21), a NADPH-dependent reductase with broad substrate specificity toward various monosaccharides, including the reduction of glucose to sorbitol.[18]
Aldose reductase has a low affinity for galactose, so under physiological conditions, galactitol is present only in traces. However, in cases of abnormal galactose accumulation, as in galactosemia, its production increases significantly.
While sorbitol can be converted to fructose in a reaction catalyzed by sorbitol dehydrogenase (EC 1.1.1.14), galactitol is not further metabolized and accumulates in the cell. This occurs because galactitol is a poorly lipid-soluble molecule, and therefore cannot efficiently cross the plasma membrane.[6]
Note: the metabolic pathway that converts glucose to fructose is known as the polyol pathway.
Galactonate
Another alternative route to the Leloir pathway leads to the oxidation of galactose to galactonate.[18]
- In the first step of the pathway, galactose is oxidized to galactono-1,4-lactone, in a reaction catalyzed by galactose dehydrogenase (EC 1.1.1.48), in which NAD+ acts as an electron acceptor and is reduced.
- In the second step, the lactone is hydrolyzed to galactonate, in a reaction that can proceed spontaneously or be catalyzed by a lactonase (EC 3.1.1.25). Galactonate can be excreted in the urine or further oxidized to β-keto-galactonate in a reaction catalyzed by β-L-hydroxy acid dehydrogenase, which also produces NADH.
- In the final step, β-keto-galactonate is decarboxylated to xylulose in a reaction catalyzed by a decarboxylase not yet identified, with release of the C1 carbon as CO2.
Finally, xylulose may enter the pentose phosphate pathway after being phosphorylated to xylulose 5-phosphate in a reaction catalyzed by xylulokinase or ATP:D-xylulose-5-phosphotransferase (EC 2.7.1.17).[22]
Galactosemia
Galactosemia is a genetic metabolic disorder caused by a defect in one of the genes encoding the enzymes of the Leloir pathway.
First described by von Reuss A. in 1908, it is characterized by the abnormal accumulation of galactonate and galactitol in various organs of the body, and is primarily caused by the accumulation of galactose-1-phosphate.[7]
The symptoms include cataracts, which often appear within the first two years of life. In the most severe forms, kidneys, liver, and brain may also be affected.
Regarding cataracts, the synthesis and accumulation of galactitol in the lens appears to be a key contributing factor, as:
- being osmotically active, galactitol causes an increase in osmotic pressure, leading to a net influx of water into the cell, which may result in osmotic lysis of the plasma membrane;[18]
- its synthesis depletes NADPH levels, thereby reducing the activity of glutathione reductase (EC 1.8.1.7). This leads to free radical accumulation and oxidative stress, which may cause cell death.[23]
To date, the standard therapy for galactosemia consists of a galactose-restricted diet.[24]
References
- ^ Pasteur L. Note sur le sucre de lait. 1856.
- ^ Berthelot M. Chimie organique fondée sur la synthèese Vol. 1, 1860. Mallet-Bachelier.
- ^ a b c Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 8th Edition. W.H. Freeman and Company, 2021.
- ^ a b c d Rosenthal M.D., Glew R.H. Medical biochemistry: human metabolism in health and disease. A John Wiley & sons, Inc., Publication, 2009.
- ^ a b Leloir L.F., de Fekete M.A., Cardini C.E. Starch and oligosaccharide synthesis from uridine diphosphate glucose. J Biol Chem 1961;236:636-41. doi:10.1016/S0021-9258(18)64280-2
- ^ a b c d e Conte F., van Buuringen N., Voermans N.C., Lefeber D.J. Galactose in human metabolism, glycosylation and congenital metabolic diseases: time for a closer look. Biochim Biophys Acta Gen Subj 2021;1865(8):129898. doi:10.1016/j.bbagen.2021.129898
- ^ a b Timson D.J. Type IV galactosemia. Genet Med 2019;21:1283-1285. doi:10.1038/s41436-018-0359-z
- ^ National Center for Biotechnology Information. PubChem Compound Summary for CID 6036, D-Galactose. https://pubchem.ncbi.nlm.nih.gov/compound/D-Galactose. Accessed Sept. 24, 2025.
- ^ a b Soderberg T. Organic chemistry with a biological emphasis. Volume II. Chemistry Publications. 2019. https://digitalcommons.morris.umn.edu/chem_facpubs/2
- ^ a b Tefsen B., Ram A.F.J., van Die I., Routier F.H. Galactofuranose in eukaryotes: aspects of biosynthesis and functional impact. Glycobiology 2012;22(4):456-469. doi:10.1093/glycob/cwr144
- ^ Flynn A. Galactosemia. Editor(s): McSweeney P.L.H., McNamara J.P. Encyclopedia of dairy sciences. 3th Edition. Academic Press, 2022;853-858. doi:10.1016/B978-0-12-818766-1.00094-5
- ^ de la Rosa L.A., Alvarez-Parrilla E., Gonzàlez-Aguilar G.A. Fruit and vegetable phytochemicals: chemistry, nutritional value, and stability. 1th Edition. Wiley J. & Sons, Inc., Publication, 2010.
- ^ Acosta P.B., Gross K.C. Hidden sources of galactose in the environment. Eur J Pediatr 1995;154(7 Suppl 2):S87-92. doi:10.1007/BF02143811
- ^ Gross K. C., Acosta P. B. Fruits and vegetables are a source of galactose: implications in planning the diets of patients with galactosaemia. J Inherit Metab Dis 1991;14(2):253-258. doi:10.1007/BF01800599
- ^ Zecca L., Mesonero J.E., Stutz A., Poirée J.C., Giudicelli J., Cursio R., Gloor S.M., Semenza G. Intestinal lactase-phlorizin hydrolase (LPH): the two catalytic sites; the role of the pancreas in pro-LPH maturation. FEBS Lett 1998;435(2-3):225-8. doi:10.1016/s0014-5793(98)01076-x
- ^ Richmond M.L., Gray J.I., and Stine C.M. Beta-galactosidase: review of recent research related to technological application, nutritional concerns, and immobilization. J Dairy Sci 1981;64:1759-1771. doi:10.3168/jds.S0022-0302(81)82764-6
- ^ a b Wright E.M., Hirayama B.A., Loo D.F. Active sugar transport in health and disease. J Intern Med 2007;261(1):32-43. doi:10.1111/j.1365-2796.2006.01746.x. PMID: 17222166
- ^ a b c d e Coelho A.I., Rubio-Gozalbo M.E., Vicente J.B., Rivera I. Sweet and sour: an update on classic galactosemia. J Inherit Metab Dis 2017;40(3):325-342. doi:10.1007/s10545-017-0029-3
- ^ Thoden J.B., Timson D.J., Reece R.J., and M. Holden H.M. Molecular structure of human galactose mutarotase. J Biol Chem 2004;279(22):23431-23437. doi:10.1074/jbc.M402347200
- ^ a b Holden H.M., Rayment I., Thoden J.B. Structure and function of enzymes of the Leloir pathway for galactose metabolism. J Biol Chem 2003;278(45):43885-43888. doi:10.1074/jbc.r300025200
- ^ Leslie N., Yager C., Reynolds R., Segal S. UDP-galactose pyrophosphorylase in mice with galactose-1-phosphate uridyltransferase deficiency. Mol Genet Metab 2005;85(1):21-7. doi:10.1016/j.ymgme.2005.01.004
- ^ Lai K., Klapa M.I. Alternative pathways of galactose assimilation: could inverse metabolic engineering provide an alternative to galactosemic patients? Metab Eng 2004 Jul;6(3):239-44. doi:10.1016/j.ymben.2004.01.001
- ^ Pintor J. Sugars, the crystalline lens and the development of cataracts. Biochem Pharmacol 2012;1:4. doi:10.4172/2167-0501.1000e119
- ^ Coelho A.I., Berry G.T., Rubio-Gozalbo M.E. Galactose metabolism and health. Curr Opin Clin Nutr Metab Care 2015;18(4):422-427. doi:10.1097/MCO.0000000000000189