In 1891, Hermann Emil Fischer, a German chemist and Nobel Laureate in Chemistry (1902), developed a systematic method for the two-dimensional representation of molecules with chiral centers (also known as chirality centers): the so-called Fischer projections, or Fischer projection formulas.[1]
Although they are two-dimensional representations, Fischer projections preserve important information about the stereochemistry of molecules.[2] While they do not reflect the actual three-dimensional shape of molecules in solution, they are still widely used by biochemists to define the stereochemistry of amino acids, carbohydrates, nucleic acids, terpenes, steroids, and other biologically relevant molecules.[3]
Contents
How to draw Fischer projections
To draw a Fischer projection of a molecule with a single chiral center (e.g., a carbon atom), the tetrahedral structure is rotated so that two groups point downward and two groups point upward. Next, draw a cross, place the chiral carbon at the intersection, and arrange the molecule so that the groups pointing downward (i.e., behind the plane of the paper) are attached to the ends of the vertical line, while the groups pointing upward (i.e., projecting out of the plane of the paper) are attached to the ends of the horizontal line.[4]
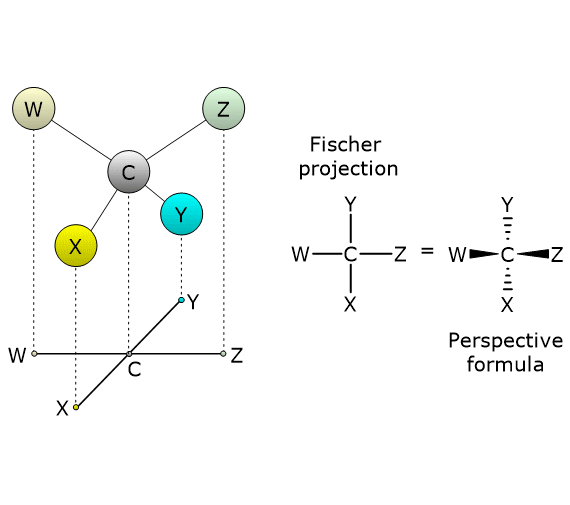
For compounds with more than one chiral center, the same procedure is applied to each asymmetric center.
A Fischer projection can also be converted into a three-dimensional representation, such as a wedge-and-dash (perspective) formula, in which the two horizontal bonds are shown as solid wedges, and the vertical bonds as dashed lines.[5]
How to manipulate Fischer projection formulas?
Since Fischer projections represent three-dimensional molecules on a two-dimensional plane, certain rules must be followed to preserve the correct configuration.[2]
- The projection must not be lifted out of the plane of the paper, as doing so would convert one enantiomer into its mirror image (the other enantiomer).
- If you rotate the projection within the plane of the paper, the configuration remains unchanged only when rotated by 180° in either direction. This is because the vertical bonds are oriented behind the plane of the paper, while the horizontal bonds project out of the plane. Conversely, a rotation of 90° or 270° in either direction inverts the configuration and converts the enantiomer into its mirror image.
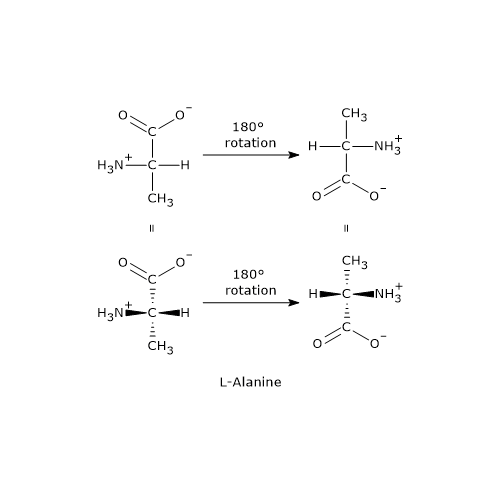
- Finally, performing an odd number of exchanges between any two groups around the chiral center also results in the conversion of one enantiomer into the other.[5]
References
- ^ Emil Fischer – Biographical. NobelPrize.org. Nobel Prize Outreach 2025. Sat. 31 May 2025. https://www.nobelprize.org/prizes/chemistry/1902/fischer/biographical/
- ^ a b Moran L.A., Horton H.R., Scrimgeour K.G., Perry M.D. Principles of Biochemistry. 5th Edition. Pearson, 2012.
- ^ Berg J.M., Tymoczko J.L., and Stryer L. Biochemistry. 5th Edition. W. H. Freeman and Company, 2002.
- ^ Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 6th Edition. W.H. Freeman and Company, 2012.
- ^ a b Solomons T.W.G., Fryhle C.B., Snyder S.A. Solomons’ organic chemistry. 12th Edition. John Wiley & Sons Incorporated, 2017.