Carbanions are ions containing a negatively charged carbon atom.
They are formed by the heterolytic cleavage of a covalent bond between a carbon atom and another atom or group.[7]
Having an unshared electron pair, they are powerful nucleophiles, and strong bases, and attack, in order to form a covalent bond, a proton or an electrophilic center, such as a polarized or positively charged center.[8]
Carbanions are extremely reactive. Therefore, they must be stabilized in order to allow their attack to the electrophilic centers.[9] Stabilization may occur by inductive effect, resonance, and may also depend on the hybridization of the carbon atom carrying the negative charge.[7][8]
They are intermediates in many enzyme-catalyzed reactions.
Contents
Heterolysis and homolysis
Considering two atoms or group, indicated as A and B, joined by a covalent bond, there are two ways to break the bond: heterolysis and homolysis.
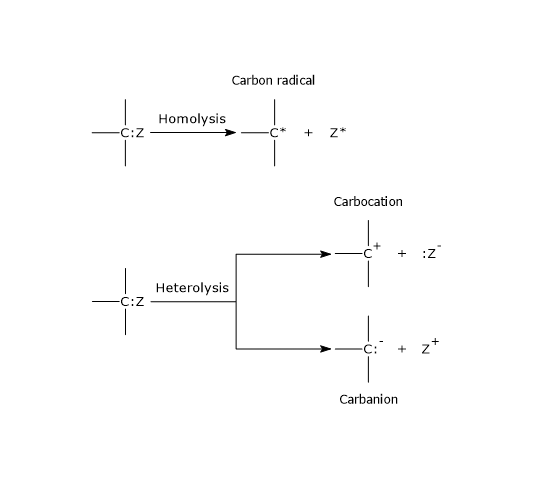
In heterolysis, the breaking of the covalent bond leads to the formation of two charged atoms, namely two ions, a cation and an anion, as both bonding electrons are taken by only one of the two previously bonded atoms, the more electronegative.
A:B → :A– + B+, if A is more electronegative than B;
A:B → A+ + :B–, if B is more electronegative than A.
In the heterolysis of a covalent bond involving a carbon atom, if both electrons are retained by the carbon atom, it will have a negative charge, therefore it is an anion, and is defined as a carbanion. On the contrary, if the carbon loses both electrons, it will have a positive charge, therefore it is a cation, and is defined as a carbocation.[5]
In homolysis, the breaking of the covalent bond between A and B leads to the formation of two free radicals, as each atom or group takes one of the two bonding electrons.[6]
Stabilization of carbanions
Carbanions are extremely reactive chemical species, and, like carbocations and free radicals, they are almost always transient intermediates in organic reactions. In order to allow their attack to the electrophilic centers, they must be stabilized. Their stabilization depends on the dispersion of the negative charge, which may occur by inductive effect, resonance, and may also depend on the s character of the hybrid orbitals of the negatively charged carbon atom.
The inductive effect is due to the presence in the molecule of one or more permanent dipoles in one or more bonds, dipoles which in turn arise from the difference in electronegativity between two groups. This difference leads to a non-uniform distribution of the bonding electrons. The inductive effect can be positive, also known as +I effect, feature of atoms or groups that tend to repel electrons, or negative, also known as –I effect, feature of atoms or groups that tend to attract electrons. The atoms or groups with the +I effect tend to decrease the stability of the carbanions, whereas those with the –I effect, therefore more electronegative, tend to stabilize them.[7]
The stability of carbanions increases when they are bound to an electrophilic structure where the unshared electron pair can delocalize by resonance, therefore a structure that acts as an electron trap or electron sink. Aromatic structures, such as the phenyl group, are particularly effective.[8]
Finally, the stability is also a function of the s character of hybrid orbitals of the negatively charged carbon atom, increasing as the percentage s character increases. Therefore it will increase going from sp3 hybridization, which has 25% s character, to sp2, with 33% s character, to sp, with 50% s character.[7]
R-CH2– < R1R2C=CH– < RC≡C–
Carbanions in enzymatic reactions
Examples of enzymatic reactions that proceed with the formation of carbanions are those catalyzed by three multienzyme complexes belonging to the family of 2-oxoacid dehydrogenases or alpha-ketoacid dehydrogenases, which are involved in the oxidative decarboxylation of ketoacids, in particular of alpha-ketoacids, briefly described below.
- The pyruvate dehydrogenase complex, which catalyzes the oxidative decarboxylation of pyruvate, the conjugate base of pyruvic acid, into acetyl-CoA, thus acting as a bridge between glycolysis and the citric acid cycle;
- The oxoglutarate dehydrogenase or alpha-ketoglutarate dehydrogenase complex, which catalyzes the oxidative decarboxylation of alpha-ketoglutarate to succinyl-CoA in step 4 of the citric acid cycle;
- The branched-chain alpha-ketoacid dehydrogenase complex, which catalyzes the oxidative decarboxylation of the branched amino acids valine, leucine and isoleucine into acetyl-CoA and succinyl-CoA. The remaining carbon skeleton can then enter the citric acid cycle.[9]
The three multienzyme complexes have very similar structures and reaction mechanisms, and their E1 subunits, which are thiamine pyrophosphate dependent enzymes, catalyze a reaction in which a carbanion intermediate is formed, whose formation and stabilization by resonance involves thiamine.[1]
Transketolase (EC 2.2.1.1) also catalyzes a reactions that involves the formation of a carbanion intermediate. This enzyme, which catalyzes steps 6 and 8 of the pentose phosphate pathway, requires thiamine pyrophosphate as a cofactor, and has a reaction mechanism similar to that of the E1 subunits of multienzyme complexes seen previously.[6]
Acetyl-CoA carboxylase (EC 6.4.1.2) is another enzyme that catalyzes a reaction that involve the formation of a carbanion intermediate. The enzyme catalyzes the committed step of de novo synthesis of fatty acids, namely, the carboxylation of acetyl-CoA to malonyl-CoA.[2]
References
- ^ Berg J.M., Tymoczko J.L., and Stryer L. Biochemistry. 5th Edition. W. H. Freeman and Company, 2002
- ^ Garrett R.H., Grisham C.M. Biochemistry. 4th Edition. Brooks/Cole, Cengage Learning, 2010
- ^ Heterolysis, in IUPAC Compendium of Chemical Terminology, 3rd ed. International Union of Pure and Applied Chemistry; 2006. Online version 3.0.1, 2019. doi:1351/goldbook.H02809
- ^ Homolysis, in IUPAC Compendium of Chemical Terminology, 3rd ed. International Union of Pure and Applied Chemistry; 2006. Online version 3.0.1, 2019. doi:1351/goldbook.H02851
- ^ Moran L.A., Horton H.R., Scrimgeour K.G., Perry M.D. Principles of Biochemistry. 5th Edition. Pearson, 2012
- ^ a b Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 6th Edition. H. Freeman and Company, 2012
- ^ a b c d Soderberg T. Organic chemistry with a biological emphasis. Volume I. Chemistry Publications. 2019
- ^ a b c Solomons T. W.G., Fryhle C.B., Snyder S.A. Solomons’ organic chemistry. 12th Edition. John Wiley & Sons Incorporated, 2017
- ^ a b Voet D. and Voet J.D. Biochemistry. 4th Edition. John Wiley J. & Sons, Inc. 2011