The phenomenon whereby two or more different chemical compounds share the same molecular formula is called isomerism, from the Greek isos meaning “equal” and meros meaning “part.” The concept and term were introduced by the Swedish scientist Jacob Berzelius in 1830.[1]
Isomerism arises from the fact that the atoms within a molecular formula can be arranged in different ways to form compounds, called isomers, that differ in their physical and chemical properties.
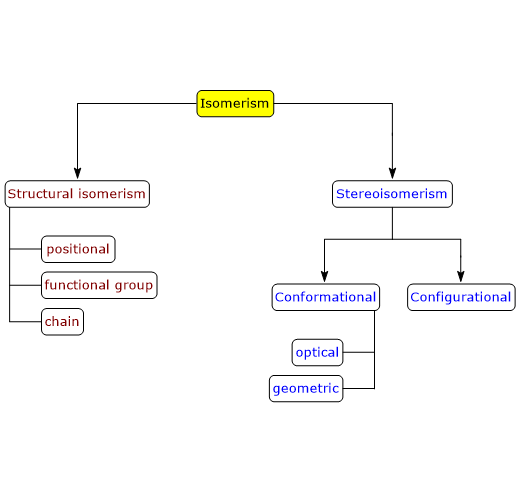
There are two main types of isomerism: structural isomerism and stereoisomerism, each of which can be further divided into subtypes.[2]
Contents
Structural isomerism
In structural isomerism, also known as constitutional isomerism, isomers differ in the way the constituent atoms are connected to each other, both in bonding sequences and atom arrangement.[3]
Several subtypes of structural isomerism exist: positional isomerism, functional group isomerism, and chain isomerism.
Positional isomers
In positional isomerism, also called position isomerism, isomers contain the same functional groups but located at different positions along the same carbon chain.[2]
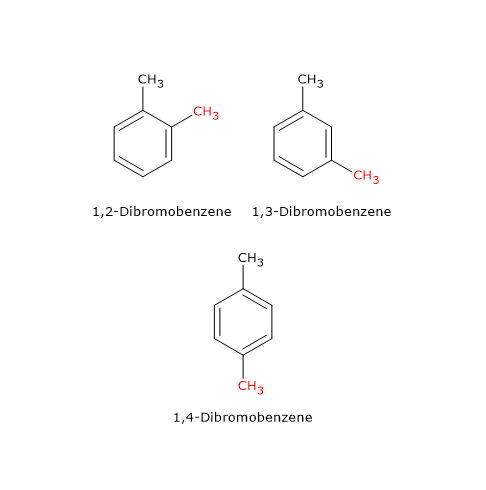
An example is the compound with molecular formula C6H4Br2, which has three isomers: 1,2-dibromobenzene, 1,3-dibromobenzene, and 1,4-dibromobenzene. These isomers differ in the position of the bromine atoms on the aromatic ring.
Another example is the compound with molecular formula C3H8O, which has two isomers: 1-propanol (also known as n-propyl alcohol) and 2-propanol (isopropyl alcohol). These differ in the position of the hydroxyl group (–OH) on the carbon chain.
Functional group isomers
Functional group isomerism, also known as functional isomerism, occurs when compounds with the same molecular formula contain different functional groups.[2]
An example is the compound with molecular formula C2H6O, which has two isomers: dimethyl ether and ethanol (also called ethyl alcohol). These isomers differ in their functional groups: an ether group (–O–) in dimethyl ether, and a hydroxyl group (–OH) in ethanol.
Chain isomers
In chain isomerism, isomers differ in the arrangement of the carbon skeleton, which may be either straight or branched.[2]
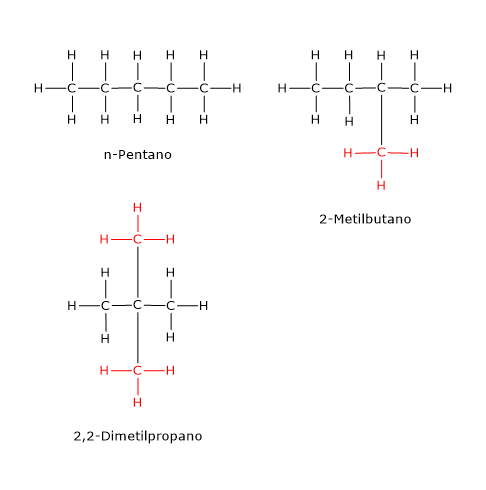
An example is the compound with the molecular formula C5H12, which has three isomers: n-pentane, 2-methylbutane (also known as isopentane), and 2,2-dimethylpropane (neopentane)
 Chain isomerism also occurs in lipids. For instance, among short-chain fatty acids, butyric acid and isobutyric acid (C4H8O2) are chain isomers, as are valeric acid, isovaleric acid, and 2-methylbutyric acid, which share the molecular formula C5H10O2.
Chain isomerism also occurs in lipids. For instance, among short-chain fatty acids, butyric acid and isobutyric acid (C4H8O2) are chain isomers, as are valeric acid, isovaleric acid, and 2-methylbutyric acid, which share the molecular formula C5H10O2.
Stereoisomerism
In stereoisomerism, isomers have the same number and types of atoms and the same bonding sequence, but differ in the spatial orientation of their atoms. Such compounds are called stereoisomers, from the Greek stereos, meaning “solid”.[4]
There are two subtypes of stereoisomerism: conformational isomerism and configurational isomerism. The latter can be further subdivided into optical isomerism and geometrical isomerism.[5]
Conformational isomerism
In conformational isomerism, stereoisomers can be interconverted by rotation around one or more single bonds (σ bonds). These rotations generate different spatial arrangements of atoms that are non-superimposable.[6] The number of possible conformations a molecule can adopt is theoretically unlimited, ranging from the lowest-energy conformation (most stable) to the highest-energy conformation (least stable). Such isomers are called conformer.[7]
For example, consider ethane C2H6. When viewed along the carbon-carbon bond using a Newman projection, the hydrogen atoms of one methyl group can adopt different orientations relative to those of the other methyl group.
- Eclipsed conformation: the hydrogen atoms of one methyl group are aligned directly behind those of the other, resulting in dihedral angles of 0°, 120°, 240°, or 360°. This is the highest-energy (and thus least stable) conformation due to increased electron repulsion.
- Staggered conformation: the hydrogen atoms of one methyl group are positioned between those of the other, giving dihedral angles of 60°, 180°, or 300°. This is the lowest-energy (most stable) conformation, as repulsions are minimized.
- Skew conformation: any intermediate orientation between eclipsed and staggered conformations.
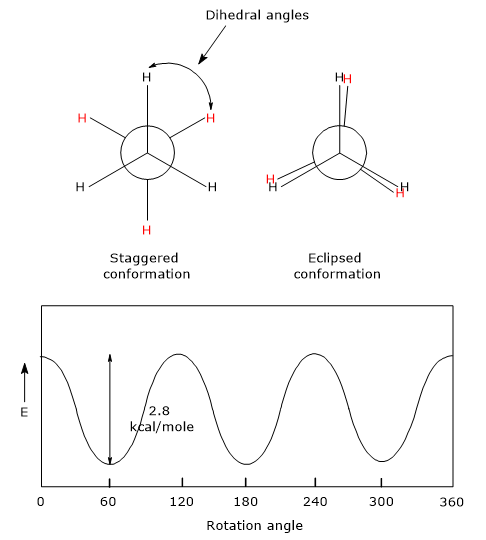
The relative stability of ethane conformers depends on the degree of overlap between the electron pairs in the C–H bonds of the two methyl groups:
- in staggered conformations, these electron pairs are as far apart as possible, minimizing repulsion;
- in eclipsed conformations, they are as close as possible, leading to increased repulsion.
The potential energy barrier between these conformations is relatively low, about 2.8 kcal/mol (11.7 kJ/mol). At room temperature, molecular kinetic energy ranges from 15 to 20 kcal/mol (62.7–83.6 kJ/mol), which is more than sufficient to overcome this barrier. As a result, free rotation occurs around the C–C bond, and individual conformers cannot be isolated.
Note: the potential energy barrier to rotation around carbon-carbon double bonds is approximately 63 kcal/mol (264 kJ/mol), corresponding to the energy required to break the π bond. This value is about three times higher than the average kinetic energy at room temperature, making free rotation impossible under normal conditions. Only at temperatures above 300 °C do molecules acquire sufficient thermal energy to break the π bond, allowing rotation around the remaining σ bond. This permits interconversion between the trans and cis isomers (see geometric isomerism).[2]
Configurational isomerism
In configurational isomerism, the interconversion between stereoisomers cannot occur through rotation around single (σ) bonds, but instead requires breaking and reforming of covalent bonds. As a result, such interconversion does not occur spontaneously at room temperature.[7]
There are two subtypes of configurational isomerism: optical isomerism and geometrical isomerism.
Optical isomers
Optical isomerism occurs in molecules that contain one or more chiral centers, which are typically tetrahedral atoms bonded to four different substituents. The chiral center is most commonly a carbon atom, but it can also be phosphorus, sulfur, or nitrogen.[6]
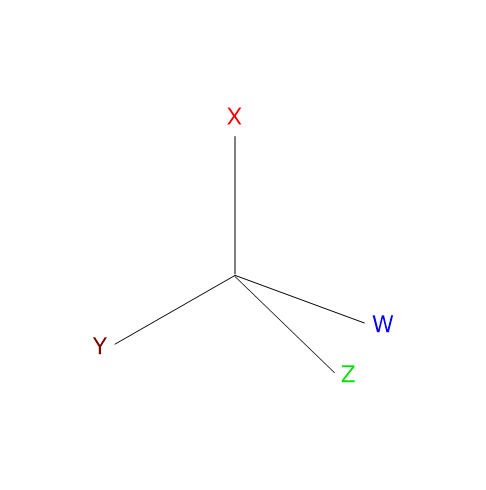 Optical isomers lack both a center and a plane of symmetry, are non-superimposable mirror images of each other, and are called enantiomers, from the Greek enántios (opposite) and meros (part). Unlike other isomers, enantiomers have identical physical and chemical properties, with two important exceptions.[8]
Optical isomers lack both a center and a plane of symmetry, are non-superimposable mirror images of each other, and are called enantiomers, from the Greek enántios (opposite) and meros (part). Unlike other isomers, enantiomers have identical physical and chemical properties, with two important exceptions.[8]
- Rotation of plane-polarized light.
One enantiomer rotates the plane of polarized light in a clockwise direction and is labeled (+), while its mirror image rotates the plane in a counterclockwise direction by the same angle and is labeled (–). This property gives rise to the term optical isomerism. - Behavior in chiral environments.
Although enantiomers are indistinguishable by most standard analytical techniques, they behave differently in chiral environments, such as the active sites of enzymes, which are themselves chiral.[9]
Note: for a molecule with n chiral centers, the maximum number of possible stereoisomers is 2n.
Geometric isomers
Geometric isomerism, also known as cis-trans isomerism, occurs when atoms cannot freely rotate due to the presence of a rigid structure, such as:
- double bonds between atoms (e.g., C=C, C=N, or N=N), where rigidity arises from the π bond;
- cyclic structures, where the ring prevents free rotation.[10]
A classic example of geometric isomerism involving a carbon–carbon double bond is stilbene (C14H12), which exists in two isomeric forms. In the cis isomer, the identical groups are on the same side of the double bond; in the trans isomer, the same groups are on opposite sides.
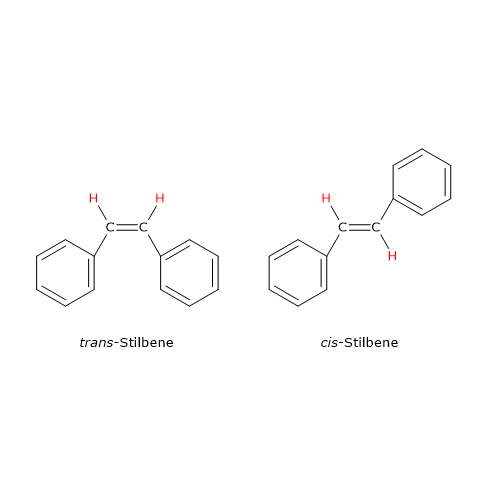
Note: the terms cis and trans derive from Latin: cis meaning “on this side of” and trans meaning “across”.
Geometric isomerism also occurs in cyclic compounds, especially when the ring contains an even number of carbon atoms and substituents are positioned opposite each other (i.e., para-substituted).[5] For example, 1,4-dimethylcyclohexane, a cycloalkane (general formula CnH2n), exists as two stereoisomers: cis-1,4-dimethylcyclohexane and trans-1,4-dimethylcyclohexane.
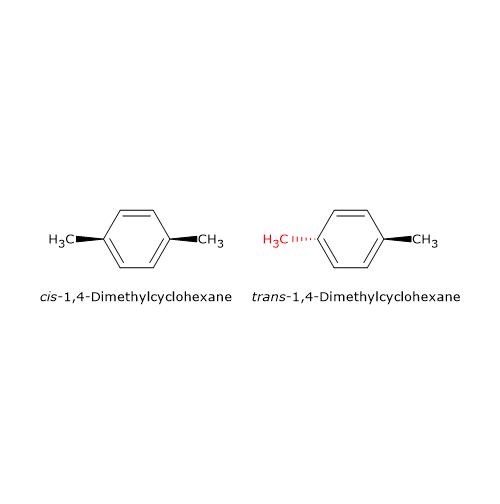
This type of stereoisomerism cannot occur if either atom involved in the restricted rotation carries two identical substituents. Why? Because converting between the cis and trans forms requires switching the positions of the groups across the rigid structure. If two groups are the same, the resulting molecule after the “switch” is identical to the original, thus, no distinct isomer is formed.[7]
Note: geometric isomers are a subclass of diastereomers, which are stereoisomers that are not mirror images of each other. Other types of diastereomers include meso compounds and non-enantiomeric optical isomers.[11]
References
- ^ Melhado E.M. Jöns Jacob Berzelius. Encyclopedia Britannica, 16 Aug. 2024. https://www.britannica.com/biography/Jons-Jacob-Berzelius. Accessed 3 June 2025.
- ^ a b c d e Solomons T.W.G., Fryhle C.B., Snyder S.A. Solomons’ organic chemistry. 12th Edition. John Wiley & Sons Incorporated, 2017.
- ^ Constitutional isomerism, in IUPAC Compendium of Chemical Terminology, 5th ed. International Union of Pure and Applied Chemistry; 2025. Online version 5.0.0, 2025. doi:10.1351/goldbook.C01285
- ^ Stereoisomerism, in IUPAC Compendium of Chemical Terminology, 5th ed. International Union of Pure and Applied Chemistry; 2025. Online version 5.0.0, 2025. doi:10.1351/goldbook.S05983
- ^ a b North M. Principles and applications of stereochemistry. 1th Edition. CRC Press, 1998.
- ^ a b Morsch L., Farmer S., Cunningham K., Sharrett Z., Shea K.M. Organic Chemistry II. Open Educational Resources: Textbooks, Smith College, Northampton, MA, 2023. https://scholarworks.smith.edu/textbooks/6
- ^ a b c Morris D.G. Stereochemistry. Royal Society of Chemistry, 2001. doi:10.1039/9781847551948
- ^ Enantiomer, in IUPAC Compendium of Chemical Terminology, 5th ed. International Union of Pure and Applied Chemistry; 2025. Online version 5.0.0, 2025. doi:10.1351/goldbook.E02069
- ^ Gogoi A., Konwer S., Zhuo G.Y. Polarimetric measurements of surface chirality based on linear and nonlinear light scattering. Front Chem 2021;8:611833. doi:10.3389/fchem.2020.611833
- ^ Hunt I. Stereochemistry. University of Calgary. Retrieved 3 November 2023.
- ^ Diastereoisomerism, in IUPAC Compendium of Chemical Terminology, 5th ed. International Union of Pure and Applied Chemistry; 2025. Online version 5.0.0, 2025. doi:10.1351/goldbook.D01679