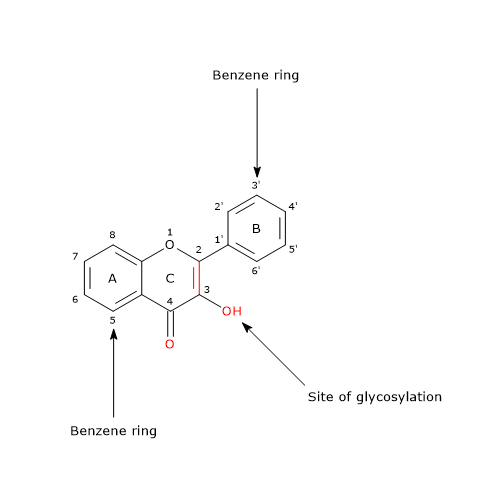
The interest on proanthocyanidins, and their content in foods, has increased as a result of the discovery, due to clinical and laboratory studies, of their anti-infectious, anti-inflammatory, cardioprotective and anticarcinogenic properties. These protective effects have been attributed to their ability to:
- act as free radical scavenger;
- inhibit the peroxidation of membrane lipids;
- act on various protein targets within the cell, modulating their activity.
Proanthocyanidins in different foods vary greatly in terms of:
- total content;
- distribution of oligomers and polymers;
- constituent catechin units and bonds between units.
In some foods, such as black beans and cashew nuts, only dimers are present, called A-type procyanidins and B-type procyanidins, whereas in most of the foods proanthocyanidins are found in a wide range of polymerizations, from 2 to 10 units or more.
Foods with the highest proanthocyanidin content are cinnamon and sorghum, which contain respectively about 8,000 and up to 4,000 mg/100 g of fresh weight (FW).
Grape seeds (Vitis vinifera) are another rich source, with a content of about 3,500 mg/100 g dry weight.
Other important sources are fruits and berries, some legumes, namely, peas and beans, red wine and to a less extent beer, hazelnuts, pistachios, almonds, walnuts and cocoa.
The coffee is not a good source.
Proanthocyanidins are not detectable in the majority of vegetables; they have been found in small concentrations in Indian pumpkin. They are not detectable also in maize, rice and wheat, while there are present in barley.
Contents
- A-type procyanidins in foods
- B-type procyanidins in foods
- Proanthocyanidins in fruits
- Proanthocyanidins in grape seeds
- References
A-type procyanidins in foods
Although many food plants contain high amounts of proanthocyanidins, only a few, such as plums, avocados, peanuts or cinnamon, contain A-type procyanidins, and none in amounts equal to cranberries (Vacciniun macrocarpon).
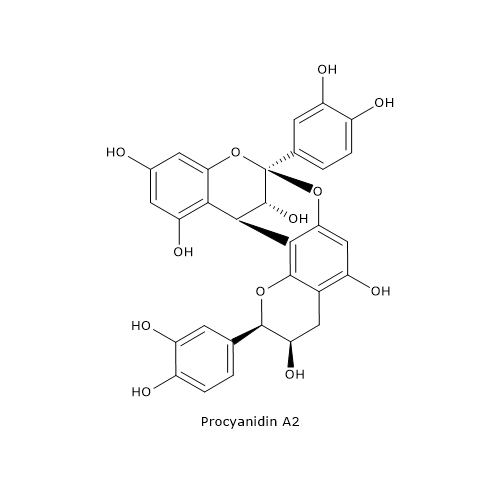
A-type procyanidins exhibit, in vitro, a capacity of inhibition of P-fimbriated Escherichia coli adhesion to uroepithelial cells greater than B-type procyanidins, adhesion which is the initial step of urogenital infections.
B-type procyanidins in foods
B-type procyanidins, consisting of catechin and/or epicatechin as constituent units, are the exclusive proanthocyanidins in at least 20 kinds of foods including blueberries (Vaccinium myrtillus), blackberries, marion berries, choke berries, grape seeds, apples, peaches, pears, nectarines, kiwi, mango, dates, bananas, Indian pumpkin, sorghum, barley, black eye peas, beans blacks, walnuts and cashews.
Proanthocyanidins in fruits
In the Western diet, fruit is the most important source of proanthocyanidins.
- The major sources are some berries, namely, blueberries, cranberries, and black currant, and plums, with a content of about 200 mg/100 g FW.
- Intermediate sources are apples, chokeberries, strawberries, and green and red grapes (60-90 mg/100 g FW).
- In other fruits the content is less than 40 mg/100 g FW.
In fruit, the most common proanthocyanidins are tetramers, hexamers, and polymers.
Good sources of proanthocyanidins are also some fruit juices.
Proanthocyanidins in grape seeds
A particularly rich source of proanthocyanidins is the seeds of grape.
Proanthocyanidins in grape seeds are only B-type procyanidins, for the most part present in the form of dimers, trimers and highly polymerized oligomers.
Grape seed proanthocyanidins are potent antioxidants and free radical scavenger, being the more effective either than vitamin E and vitamin C or ascorbic acid.
In vivo and in vitro experiments support the idea that proanthocyanidins, and in particular those from grape seeds, can act as anti-carcinogenic agents; it seems that they are involved, in cancer cells, in:
- reduction of cell proliferation;
- increase of apoptosis;
- cell cycle arrest;
- modulation of the expression and activity of NF-kB and NF-kB target genes.
References
- de la Rosa L.A., Alvarez-Parrilla E., Gonzàlez-Aguilar G.A. Fruit and vegetable phytochemicals: chemistry, nutritional value, and stability. 1th Edition. Wiley J. & Sons, Inc., Publication, 2010
- Gu L., Kelm M.A., Hammerstone J.F., Beecher G., Holden J., Haytowitz D., Gebhardt S., and Prior R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J Nutr 2004;134(3):613-617. doi:10.1093/jn/134.3.613
- Han X., Shen T. and Lou H. Dietary polyphenols and their biological significance. Int J Mol Sci 2007;9:950-988. doi:10.3390/i8090950
- Manach C., Scalbert A., Morand C., Rémésy C., and Jime´nez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004;79(5):727-747. doi:10.1093/ajcn/79.5.727
- Nandakumar V., Singh T., and Katiyar S.K. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett 2008;269(2):378-387. doi:10.1016/j.canlet.2008.03.049
- Ottaviani J.I., Kwik-Uribe C., Keen C.L., and Schroeter H. Intake of dietary procyanidins does not contribute to the pool of circulating flavanols in humans. Am J Clin Nutr 2012;95:851-858. doi:10.3945/ajcn.111.028340
- Santos-Buelga C. and Scalbert A. Proanthocyanidins and tannin-like compounds: nature, occurrence, dietary intake and effects on nutrition and health. J Sci Food Agr 2000;80(7):1094-1117. doi:10.1002/(SICI)1097-0010(20000515)80:7<1094::AID-JSFA569>3.0.CO;2-1
- Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010;2:1231-1246. doi:10.3390/nu2121231
- Wang Y.,Chung S., Song W.O., and Chun O.K. Estimation of daily proanthocyanidin intake and major food sources in the U.S. diet. J Nutr 2011;141(3):447-452. doi:10.3945/jn.110.133900