Trehalose is a disaccharide composed of two α-D-glucose molecules joined by an α, α-(1→1) glycosidic bond.[1]
It was discovered in an ergot fungus of the genus Claviceps by H.A.L. Wiggers in 1832.[2] Twenty-seven years later, in 1859, M. Berthelot isolated the disaccharide from Trehala manna, a cocoon-shaped substance produced by larval activity of weevils of the Curculionidae family on certain Echinops species, from which the name.[3]
Trehalose is a non-reducing sugar with a low chemical reactivity. In nature, sucrose is the other widely distributed non-reducing sugar.[4]
It is found in all major groups of organisms, including bacteria, fungi, yeasts, algae, plants, and invertebrates, but it is absent in vertebrates.[5]
Dietary sources include certain species of fungi, yeasts, crustaceans, honey, and processed foods to which it may be added as an additive.[6]
It serves various functions, such as carbohydrate storage, energy source, membrane lipid component, signaling molecule between plants and symbiotic or pathogenic microorganisms, and protection against abiotic stress.[7] In its phosphorylated form, it is involved in the regulation of carbohydrate metabolism in plants.[8]
Mammals can utilize exogenous trehalose as a source of carbon and energy. Its intestinal digestion is mediated by the enzyme trehalase (EC 3.2.1.28), which catalyzes the hydrolysis of trehalose into two glucose molecules.[9]
Contents
- Chemical properties
- Dietary sources
- Biosynthesis
- Trehalose in plants
- Functions
- Molecular mechanisms of protective potential
- Trehalose digestion
- Trehalase deficiency
- References
Chemical properties
Like other disaccharides such as lactose, sucrose, and maltose, trehalose has the molecular formula C12H22O11 and a molecular weight of 342.30 g/mol.
According to IUPAC nomenclature, its systematic name is α-D-gluco-hexopyranosyl α-D-gluco-hexopyranoside.[1]
Because the α, α-(1→1) glycosidic bond forms between the anomeric carbons of both glucose units, it cannot adopt an open-chain form with a free aldehyde group in solution. As a result, it does not act as a reducing agent. Therefore, this disaccharide is classified as a non-reducing sugar and exhibits low chemical reactivity.[10]
At room temperature, it appears as white, orthorhombic crystals.
Its melting point is 203 °C (397.4 °F; 476.15 K)
Trehalose provides approximately 4 kcal/g of energy.[1]
Its sweetness is about 45% that of sucrose.[11]
Dietary sources
Dietary sources of trehalose include certain species of fungi (e.g. Amanita spp.), particularly when young, as well as yeasts, crustaceans, and honey. In 2000, the FDA approved its use as a food additive, allowing its inclusion in products such as flavor enhancers and preservatives. Since it occurs only in small amounts in a limited number of unprocessed foods, processed foods, such as frozen shrimp, vacuum-packed fish, breakfast cereals, and baked goods, have become its primary dietary sources.[6]
Biosynthesis
In prokaryotes, five biosynthetic pathways for trehalose have been identified, although only one, likely the most widespread and the canonical pathway, is also present in eukaryotes.[12][13] This pathway is described below.
It involves two enzymatic steps.
In the first step, catalyzed by trehalose 6-phosphate synthase (EC 2.4.1.15), UDP-glucose and glucose 6-phosphate condense to form trehalose 6-phosphate.
In the second step, trehalose 6-phosphate is dephosphorylated to trehalose through a hydrolysis reaction catalyzed by trehalose 6-phosphate phosphatase (EC 3.1.3.12).
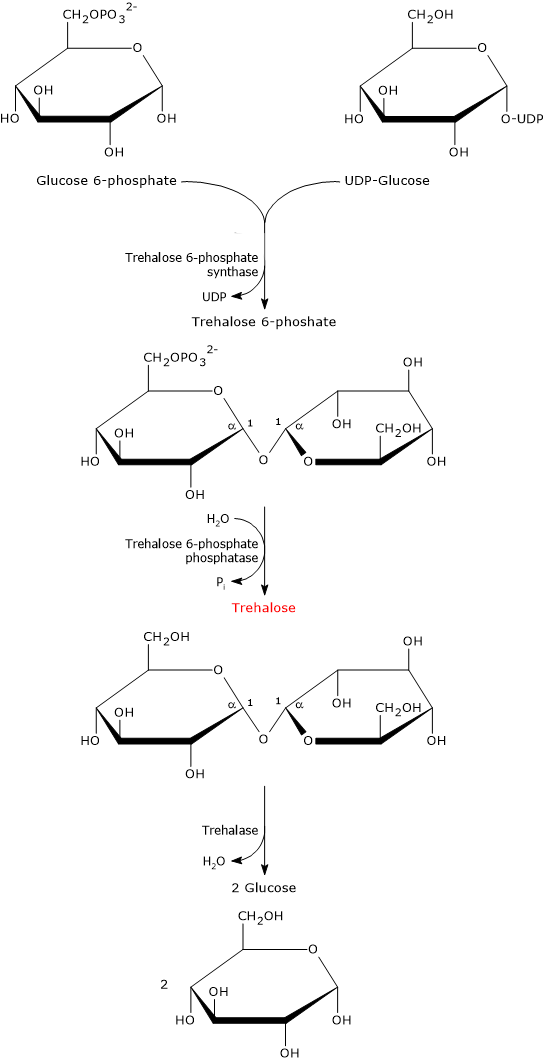
The biosynthesis of this disaccharide is strongly induced in response to abiotic stress. The importance of this disaccharide is underscored by the fact that some organisms possess multiple metabolic pathways for its synthesis.[7]
No gene involved in trehalose biosynthesis or storage has been identified in vertebrates. It does not appear that vertebrates lost the ability to synthesize this disaccharide over the course of evolution; rather, they seem never to have acquired it.[5]
Trehalose in plants
Trehalose is the predominant disaccharide found in growing plants.[14]
It was first detected in plants in 1913 in Selaginella lepidophylla, also known as the resurrection fern or false rose of Jericho, a desiccation-tolerant vascular plant.[15] It was later identified in green algae, mosses, liverworts (non-vascular plants of the division Marchantiophyta), and ferns.[16]
Its presence in angiosperms was long believed to result from microbial or bacterial contamination, or from analytical artifacts. This view began to change in the 1990s, when large amounts of trehalose were found in desiccation-tolerant angiosperms, such as Myrothamnus flabellifolius and Sporobolus spp., amounts too substantial to be explained by microbial contamination alone.[17][18]
Despite this, trehalose and its metabolism were still considered insignificant, or even absent, in most angiosperms until two pivotal observations changed this view.
In 1998, in Arabidopsis thaliana, a desiccation-intolerant plant of the Brassicaceae family widely used as a model organism for higher plants, genes were identified that encode catalytically active trehalose 6-phosphate synthase and trehalose 6-phosphate phosphatase.[19][20] Furthermore, the importance of trehalose metabolism in angiosperms became evident through experiments in which fungal or bacterial enzymes were introduced to genetically engineer trehalose production. The resulting transgenic plants exhibited a wide range of phenotypic anomalies, including delayed senescence and altered leaf morphology.[21] These findings led to two important conclusions regarding angiosperms:
- the capacity to synthesize this disaccharide is not limited to resurrection plants;
- Disruption of trehalose metabolism has broad effects on overall metabolism and development.[22]
To date, genes encoding trehalose 6-phosphate synthase and trehalose 6-phosphate phosphatase have been identified in all major plant taxa, suggesting that the ability to synthesize trehalose is universal across the plant kingdom.[14]
Functions
Trehalose serves multiple functions across a wide range of organisms. Some of these roles are shared among various groups, while others are specific to particular taxa.
- In plants, insects, nematodes, and bacteria, it plays a key role in the adaptive response to abiotic stresses such as extreme temperatures, osmotic pressure and salinity fluctuations, nutrient deprivation, and desiccation.[23] By accumulating within cells, trehalose helps mitigate damage to biological macromolecules, aiding in survival under adverse conditions.[24] In plants, its accumulation during stress is associated with the transcriptional activation of genes encoding biosynthetic enzymes or with the inhibition of trehalase activity.[25] Once the stress conditions subside, trehalose levels return to normal.[8]
- Trehalose can also function as a storage carbohydrate and a molecule for carbon transport. In certain bacterial spores, it can accumulate to about 25% of the spore’s dry weight.[26]
Some bacteria utilize it as an exogenous carbon source.[5]
In fungi and yeasts, it is stored during dormancy.[27] - In insects, the disaccharide is the predominant sugar in the hemolymph, constituting 80–90% of the circulating carbohydrates, and serves as a rapidly available energy source for flight.[16][28]
- Trehalose is also considered a potential signaling metabolite involved in interactions between plants and both symbiotic and pathogenic microorganisms, as well as herbivorous insects.[14] Moreover, some bacteria and fungi depend on its metabolism for their infectivity.[29][30]
Other roles
Trehalose is a structural component of lipids found in the cell walls of bacteria belonging to the genera Corynebacterium and Mycobacterium.[31] For example, the cell wall of Mycobacterium tuberculosis contains trehalose-derived glycolipids, such as sulfolipids, diacyl-, triacyl-, and polyacyltrehaloses, in which fatty acids are esterified to the hydroxyl groups of the disaccharide rather than to those of glycerol.[32]
Trehalose is also thought to protect unsaturated fatty acids with cis double bonds from oxidative damage.[33] Additionally, it has been proposed that trehalose can prevent protein aggregation by interacting with cis double bonds present in the side chains of aromatic amino acids and by limiting the acetylation of the ε-amino group on lysine residues, which would otherwise increase protein hydrophobicity.[33][34]
In insects, trehalose plays a crucial role in growth and development, accounting for approximately 20% of the total carbohydrate pool during certain developmental stages.[5]
Trehalose 6-phosphate is essential for both embryonic and vegetative development in plants, as well as for sucrose and starch metabolism.[35] It is hypothesized that the primary function of trehalose 6-phosphate is to signal and regulate sucrose levels, acting as a negative feedback regulator.[36]
Molecular mechanisms of protective potential
Trehalose is involved in the response to abiotic stresses in different organisms.
Its protective potential is believed to arise from multiple, synergistic mechanisms that stem from its unique chemical properties, particularly its status as a non-reducing sugar.[7]
Due to its low chemical reactivity, it does not readily interact with other cellular molecules such as proteins, DNA, or RNA.[10]
Its high hydrophilicity enables it to form strong hydrogen bonds with water, stronger than those between water molecules themselves. While other disaccharides can also displace water, trehalose exhibits a higher hydration number, meaning it associates with more water molecules than other disaccharides.[11][33]
This combination of low reactivity and high hydrophilicity makes it compatible with cellular metabolism, even at high intracellular concentrations.[18][37]
The α, α-(1→1) glycosidic bond in trehalose is more flexible than the glycosidic bonds found in other disaccharides, allowing the molecule to more easily conform to the polar groups of biological macromolecules.[10]
Another important feature is the α, α-(1→1) glycosidic bond’s high resistance to enzymatic and acid hydrolysis.[8]
However, what most clearly distinguishes trehalose from other disaccharides is its remarkable ability to form a glass-like matrix around biological molecules. This glassy structure remains stable even under high temperatures and during desiccation. Trehalose appears to replace water molecules that would normally hydrogen-bond with proteins and other macromolecules, thereby preserving their native three-dimensional structure and functional integrity in the absence of water.[11]
Trehalose digestion
In mammals, most carbohydrate digestion occurs in the duodenum. Pancreatic alpha-amylase and hydrolases located in the brush border of enterocytes hydrolyze disaccharides, oligosaccharides, and polysaccharides into their constituent monosaccharides, namely glucose, fructose, and galactose. These monosaccharides are then absorbed.
The α, α-(1→1) glycosidic bond in trehalose is cleaved by the enzyme trehalase in a hydrolytic reaction, releasing two molecules of glucose for each molecule of the disaccharide.[9]
Trehalase deficiency
There are two genes in the human genome that encode trehalase isoforms.[38]
Mutations in these genes can result in the production of non-functional or poorly functioning enzymes. As a consequence, undigested trehalose remains in the intestine, where it can cause osmotic diarrhea. Once it reaches the colon, it may be metabolized by gut microbiota, part of the broader human microbiota, leading to the production of excessive amounts of gas, short-chain fatty acids (mainly acetic, propionic, and butyric acids), and alcohols. This process contributes to the symptoms of trehalose intolerance, including osmotic-fermentative diarrhea, malabsorption, and various abdominal complaints.[39][40]
Currently, there is no cure for trehalase deficiency. The only available treatment is to avoid or limit the intake of foods containing the disaccharide
References
- ^ a b c National Center for Biotechnology Information. PubChem Compound Summary for CID 7427, Trehalose. https://pubchem.ncbi.nlm.nih.gov/compound/Trehalose. Accessed Sept. 25, 2025.
- ^ Wiggers H.A.L. Untersuchung über das Mutterkorn, Secale cornutum. Annalen der Pharmacie 1832;1(2):129-182. doi:10.1002/jlac.18320010202
- ^ Berthelot M. Nouvelles recherches sur les corps analogues au sucre de canne. Annales de Chimie et de Physique 1859 3a serie;55:269-282.
- ^ Yoon Y.S., Cho E.D., Jung Ahn W., Won Lee K., Lee S.J., Lee H.J. Is trehalose an autophagic inducer? Unraveling the roles of non-reducing disaccharides on autophagic flux and alpha-synuclein aggregation. Cell Death Dis 2017;8(10):e3091. doi:10.1038/cddis.2017.501
- ^ a b c d Argüelles J.C. Why can’t vertebrates synthesize trehalose? J Mol Evol 2014;79:111-116. doi:10.1007/s00239-014-9645-9
- ^ a b Evaluations of the joint FAO/WHO expert committee on food additives (JECFA). alpha,alpha-Trehalose. Evaluation year: 2000. https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/4067
- ^ a b c Vanaporn M., Titball R.W. Trehalose and bacterial virulence. Virulence 2020 Dec;11(1):1192-1202. doi:10.1080/21505594.2020.1809326
- ^ a b c Lee H.J., Yoon Y.S., Lee S.J. Mechanism of neuroprotection by trehalose: controversy surrounding autophagy induction. Cell Death Dis 2018;9(7):712. doi:10.1038/s41419-018-0749-9
- ^ a b Rosenthal M.D., Glew R.H. Medical biochemistry: human metabolism in health and disease. A John Wiley & sons, Inc., Publication, 2009.
- ^ a b c Colaco C., Kampinga J., Roser B. Amorphous stability and trehalose. Science 1995;268(5212):788. doi:10.1126/science.7754360
- ^ a b c Jain N.K., Roy I. Effect of trehalose on protein structure. Protein Sci 2009;18(1):24-36. doi:10.1002/pro.3
- ^ Avonce N., Mendoza-Vargas A., Morett E., Iturriaga G. Insights on the evolution of trehalose biosynthesis. BMC Evol Biol 2006;6:109. doi:10.1186/1471-2148-6-109
- ^ Cabib E., Leloir L.F. The biosynthesis of trehalose phosphate. J Biol Chem 1958;231(1):259-75.
- ^ a b c Lunn J.E., Delorge I., Figueroa C.M., Van Dijck P., Stitt M. Trehalose metabolism in plants. Plant J 2014;79(4):544-67. doi:10.1111/tpj.12509
- ^ Anselmino O. and Gilg E. Uber das Vorkommen von Trehalose in Selaginella lepidophylla. Ber Deut Pharm Ges 1913;23:326-330.
- ^ a b Elbein A.D. The metabolism of alpha,alpha-trehalose. Adv Carbohydr Chem Biochem 1974;30:227-56. doi:10.1016/s0065-2318(08)60266-8
- ^ Drennan P.M., Smith M.T., Goldsworthy D., Van Staden J. The occurrence of trehalose in the leaves of the desiccation-tolerant angiosperm Myrothamnus flabellifolius Welw. J Plant Physiol 1993;142(4):493-6. doi:10.1016/S0176-1617(11)81257-5
- ^ Iturriaga G., Gaff D.F., Zentella R. New desiccation-tolerant plants, including a grass, in the central highlands of Mexico, accumulate trehalose. Australian Journal of Botany 2000;48(2):153-8. doi:10.1071/BT98062
- ^ Blazquez M., Santos E., Flores C., Martinez-Zapater J., Salinas J., Gancedo C. Isolation and molecular characterization of the Arabidopsis TPS1 gene, encoding trehalose-6-phosphate synthase. Plant J 1998;13(5):685-9. doi:10.1046/j.1365-313x.1998.00063.x
- ^ Vogel G., Aeschbacher R.A., Muller J., Boller T., Wiemken A. Trehalose-6-phosphate phosphatases from Arabidopsis thaliana: identification by functional complementation of the yeast tps2 mutant. Plant J 1998;13:673-683. doi:10.1046/j.1365-313X.1998.00064.x
- ^ Goddijn O.J., Verwoerd T.C., Voogd E., Krutwagen R.W., de Graaf P.T., van Dun K., Poels J., Ponstein A.S., Damm B., Pen J. Inhibition of trehalase activity enhances trehalose accumulation in transgenic plants. Plant Physiol 1997;113(1):181-90. doi:10.1104/pp.113.1.181
- ^ Figueroa C.M., Lunn J.E. A tale of two sugars: trehalose 6-phosphate and sucrose. Plant Physiol 2016;172(1):7-27. doi:10.1104/pp.16.00417
- ^ Crowe J.H., Hoekstra F.A., Crowe L.M. Anhydrobiosis. Annu Rev Physiol 1992;54:579-99. doi:10.1146/annurev.ph.54.030192.003051
- ^ Richards A.B., Krakowka S., Dexter L.B., Schmid H., Wolterbeek A.P., Waalkens-Berendsen D.H., Shigoyuki A., Kurimoto M. Trehalose: a review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem Toxicol 2002;40(7):871-98. doi:10.1016/s0278-6915(02)00011-x
- ^ Fernandez O., Béthencourt L., Quero A., Sangwan R.S., Clément C. Trehalose and plant stress responses: friend or foe? Trends Plant Sci 2010;15(7):409-17. doi:10.1016/j.tplants.2010.04.004
- ^ McBride M.J., Ensign J.C. Effects of intracellular trehalose content on Streptomyces griseus spores. J Bacteriol 1987;169(11):4995-5001. doi:10.1128/jb.169.11.4995-5001.1987
- ^ Barton J.K., Den Hollander J.A., Hopfield J.J., Shulman R.G. 13C nuclear magnetic resonance study of trehalose mobilization in yeast spores. J Bacteriol 1982;151(1):177-85. doi:10.1128/jb.151.1.177-185.1982
- ^ Wyatt G.R., Kale G.F. The chemistry of insect hemolymph. II. Trehalose and other carbohydrates. J Gen Physiol 1957;40(6):833-47. doi:10.1085/jgp.40.6.833
- ^ Wilson R.A., Jenkinson J.M., Gibson R.P., Littlechild J.A., Wang Z.Y., Talbot N.J. Tps1 regulates the pentose phosphate pathway, nitrogen metabolism and fungal virulence. EMBO J 2007 Aug;26(15):3673-85. doi:10.1038/sj.emboj.7601795
- ^ Djonović S., Urbach J.M., Drenkard E., Bush J., Feinbaum R., Ausubel J.L., Traficante D., Risech M., Kocks C., Fischbach M.A., Priebe G.P., Ausubel F.M. Trehalose biosynthesis promotes Pseudomonas aeruginosa pathogenicity in plants. PLoS Pathog 2013;9(3):e1003217. doi:10.1371/journal.ppat.1003217
- ^ Iturriaga G., Suárez R., Nova-Franco B. Trehalose metabolism: from osmoprotection to signaling. Int J Mol Sci 2009;10(9):3793-3810. doi:10.3390/ijms10093793
- ^ Kalscheuer R., Koliwer-Brandl H. Genetics of mycobacterial trehalose metabolism. Microbiol Spectr 2014;2(3). doi:10.1128/microbiolspec.MGM2-0002-2013
- ^ a b c Furuki T., Oku K., Sakurai M. Thermodynamic, hydration and structural characteristics of alpha,alpha-trehalose. Front Biosci (Landmark Ed). 2009;14(9):3523-35. doi:10.2741/3468
- ^ Moruno Algara M., Kuczyńska-Wiśnik D., Dębski J., Stojowska-Swędrzyńska K., Sominka H., Bukrejewska M., Laskowska E. Trehalose protects Escherichia coli against carbon stress manifested by protein acetylation and aggregation. Mol Microbiol 2019;112(3):866-880. doi:10.1111/mmi.14322
- ^ Schluepmann H., Pellny T., van Dijken A., Smeekens S. and Paul M. Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. Proc Natl Acad Sci 2003;100(11):6849-6854. doi:10.1073/pnas.1132018100
- ^ Yadav U.P., Ivakov A., Feil R., Duan G.Y., Walther D., Giavalisco P., Piques M., Carillo P., Hubberten H.M., Stitt M., Lunn J.E. The sucrose-trehalose 6-phosphate (Tre6P) nexus: specificity and mechanisms of sucrose signalling by Tre6P. J Exp Bot 2014;65(4):1051-68. doi:10.1093/jxb/ert457
- ^ Lin Q., Gong J., Zhang Z., Meng Z., Wang J., Wang S., Sun J., Gu X., Jin Y., Wu T., Yan N., Wang Y., Kai L., Jiang J., Qi S. The Arabidopsis thaliana trehalose-6-phosphate phosphatase gene AtTPPI regulates primary root growth and lateral root elongation. Front Plant Sci 2023;13:1088278. doi:10.3389/fpls.2022.1088278
- ^ TREH trehalase [Homo sapiens]. Gene ID: 11181, updated on 19-Aug-2025. https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=11181
- ^ Holtug K., Clausen M.R., Hove H., Christiansen J., Mortensen P.B. The colon in carbohydrate malabsorption: short-chain fatty acids, pH, and osmotic diarrhoea. Scand J Gastroenterol 1992;27(7):545-52. doi:10.3109/00365529209000118
- ^ Arola H., Koivula T., Karvonen A.L., Jokela H., Ahola T., Isokoski M. Low trehalase activity is associated with abdominal symptoms caused by edible mushrooms. Scand J Gastroenterol 1999;34(9):898-903. doi:10.1080/003655299750025372