Carbohydrates, together with lipids, proteins, and nucleic acids, constitute one of the four major classes of biologically essential organic molecules found in all living organisms. Along with lipids and proteins, they are also one of the three macronutrients required to sustain physiological functions and maintain health.
From a biochemical standpoint, carbohydrates originate primarily from photosynthesis and play a central role in both plant and animal metabolism. In human nutrition, carbohydrates are the main source of readily available energy and are a defining feature of traditional dietary patterns, such as the Mediterranean diet.
The nutritional and physiological relevance of carbohydrates is closely linked to their chemical structure and degree of polymerization. For this reason, carbohydrates can be classified according to chemical, physiological, and nutritional criteria. As with lipids and protein classification, these approaches provide a useful framework for understanding the diversity of carbohydrates and their biological roles.
Carbohydrates perform a wide range of essential functions. They act as immediate and stored sources of energy, contribute to normal lipid metabolism, and support tissues with specific glucose requirements. In addition, carbohydrates participate in detoxification processes, cell–cell recognition and signaling, and form the structural backbone of nucleic acids.
Summary: Key Points
- Definition: organic compounds defined as polyhydroxy aldehydes or ketones.
- Chemical classification: divided into monosaccharides (single units), oligosaccharides (2 to 10–20 units), and polysaccharides (> 20 units) according to the degree of polymerization, and into reducing and non-reducing sugars based on chemical reactivity.
- Physiological and nutritional classification: categorized as available carbohydrates (digested and absorbed) or unavailable carbohydrates (fermented by the gut microbiota).
- Biological functions: provision of immediate and stored energy (≈4 kcal/g), structural support, participation in detoxification and cell signaling, protein-sparing effect, normal lipid metabolism, and maintenance of DNA and RNA structural integrity.
Contents
- Chemical features of carbohydrates
- Chemical classification of carbohydrates
- Physiological and nutritional classification of carbohydrates
- Functions of carbohydrates
- References
Chemical features of carbohydrates
Carbohydrates, also called carbs, are defined as aldehyde or ketone compounds containing a variable number of hydroxyl groups. For this reason, they are also referred to as polyhydroxy aldehydes or ketones; in the case of monosaccharides, these are specifically termed aldoses or ketoses.
Many, but not all, carbohydrates have the general chemical formula (CH2O)n; however, only molecules with n ≥ 3 are conventionally classified as carbohydrates. Some carbohydrates, in addition to carbon, hydrogen, and oxygen, may also contain nitrogen or sulfur.
Chemical classification of carbohydrates
From a chemical standpoint, carbohydrates can be classified according to several structural criteria, most notably the number of constituent units and the degree of polymerization. Based on the number of constituent units, carbohydrates are divided into three major classes: monosaccharides, oligosaccharides, and polysaccharides. Alternatively, based on their degree of polymerization, carbohydrates are classified as simple or complex.
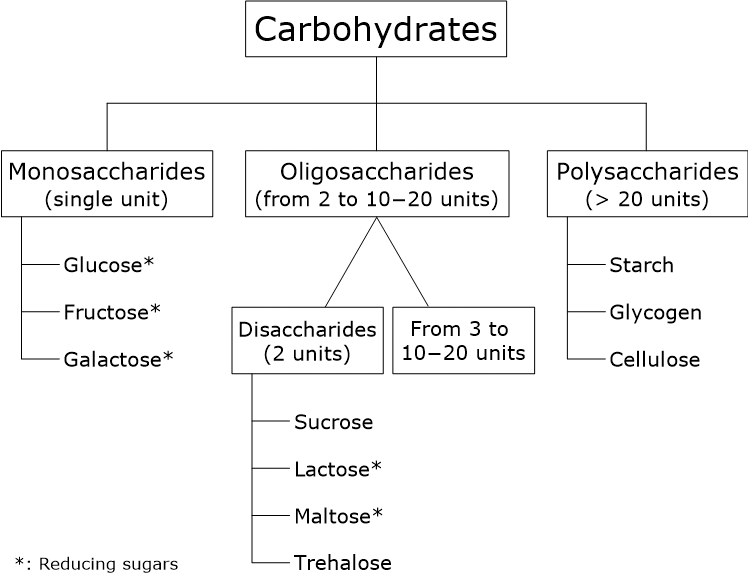
Monosaccharides
Monosaccharides, or simply sugars, consist of a single polyhydroxy aldehyde or ketone unit. The most abundant monosaccharide is D-glucose, which is an aldose and also known as dextrose. Other monosaccharides important in human nutrition are fructose, a ketose, and galactose, an aldose.
Oligosaccharides
Oligosaccharides are composed of short chains of monosaccharide units, typically ranging from 2 to 10–20, linked together by chemical bonds known as glycosidic bonds. The most abundant oligosaccharides are disaccharides, which are formed by two monosaccharides. In the human diet, the most important disaccharides are sucrose (table sugar), lactose, maltose, and trehalose. Within cells, many oligosaccharides composed of three or more units are not found as free molecules but are covalently linked to lipids or proteins, forming glycoconjugates.
Polysaccharides
Polysaccharides are polymers consisting of more than 20 and up to several thousand monosaccharide units. They differ from one another in the type of monosaccharides present, the length and degree of branching of their chains, and the nature of the glycosidic linkages between units. While numerous types of polysaccharides are found in the plant kingdom, only a limited number are present in vertebrates.
Polysaccharides can be classified as:
- Homopolysaccharides, when they contain only one type of monosaccharide, such as starch (which consists of a mixture of two polysaccharides, amylose and amylopectin), glycogen, and cellulose;
- Heteropolysaccharides, which contain two or more different types of monosaccharides, such as hyaluronic acid.
Note: the term saccharide derives from the Greek word sakcharon, meaning “sugar”.
Degree of polymerization
Based on their degree of polymerization, carbohydrates can be classified as simple or complex.
- Simple carbohydrates include monosaccharides and disaccharides, commonly referred to as sugars, as well as tri- and tetrasaccharides, which are considered short-chain oligosaccharides.
- Complex carbohydrates are represented by polysaccharides, which consist of long chains of monosaccharide units.
Reducing and non-reducing sugars
Carbohydrates can also be classified according to their chemical reactivity, specifically their ability to act as reducing agents.This property depends on the presence of a free anomeric carbon, the carbon atom derived from the carbonyl group (aldehyde or ketone) during cyclization; its configuration gives rise to anomerism.
- Reducing sugars: these carbohydrates possess a free, or potentially free, anomeric carbon in the form of a hemiacetal or hemiketal. As a result of anomerism, the cyclic structure can equilibrate with the open-chain form in aqueous solution, transiently exposing a reactive aldehyde or ketone group. All monosaccharides and many disaccharides, such as lactose and maltose, are reducing sugars.
- Non-reducing sugars: in these carbohydrates, the anomeric carbons of the constituent monosaccharide units are involved in glycosidic bonds. Because anomerism is no longer possible and the cyclic structure cannot reopen to expose a reactive group, these sugars cannot act as reducing agents. Common examples include sucrose and trehalose. It is worth noting that while large polysaccharides like starch and glycogen technically possess one reducing end, their enormous molecular size and the fact that nearly all their anomeric carbons are locked in glycosidic bonds mean they do not exhibit significant reducing properties in standard chemical tests.
Physiological and nutritional classification of carbohydrates
A further classification of carbohydrates is based on their ability to be directly used for energy production and includes:
- available carbohydrates, such as glucose, fructose, and galactose among monosaccharides; sucrose, lactose, maltose, and maltodextrin among oligosaccharides; and starch and glycogen among polysaccharides;
- unavailable carbohydrates, such as xylose (a monosaccharide), lactulose (a disaccharide), and raffinose (a trisaccharide), dietary fiber (including cellulose, hemicellulose, and pectins), and resistant or non-digestible starches.
Carbohydrates belonging to this latter group, even when ingested, are neither digested nor absorbed in the small intestine. Instead, they are fermented by the gut microbiota, which is part of the broader human microbiota. This fermentation leads to the production of short-chain fatty acids and to the subsequent release of a limited amount of energy.
Functions of carbohydrates
Carbohydrates perform a variety of physiological and biological functions that extend beyond their structural classification. From an energetic and metabolic perspective, they play a central role in fueling cellular activities, regulating the metabolism of other macronutrients, and supporting tissues with specific glucose requirements. In addition, carbohydrates contribute to detoxification mechanisms, participate in molecular recognition processes, and provide mechanical support in both unicellular and multicellular organisms. These diverse roles reflect the functional versatility of carbohydrates, whose main biological functions are outlined below.
| Functional category | Typical examples |
|---|---|
| Energy production and storage | Glucose for ATP synthesis; starch in plants; glycogen in animals |
| Protein-sparing effect | Dietary carbohydrates reducing the use of proteins for energy |
| Role in lipid metabolism | Carbohydrates enabling fatty acid oxidation; excess glucose converted to triglycerides in the liver |
| Glucose-dependent tissues | Central nervous system, red blood cells |
| Structural role in nucleic acids | Ribose in RNA; deoxyribose in DNA |
| Detoxification processes | Glucuronic acid conjugation of hormones, bilirubin, drugs, and toxins in the liver |
| Cell recognition and signaling | Glycoproteins and glycolipids mediating immune interactions, leukocyte adhesion, sperm–oocyte recognition |
| Structural and extracellular support | Cellulose in plant cell walls; chitin in arthropod exoskeletons; heteropolysaccharides in extracellular matrices |
| Technological and sensory functions | Food texture, viscosity, sweetness, browning reactions, flavor enhancement |
Energy production and storage
Carbohydrates serve as primary substrates for both energy production and energy storage. Starch in plants and glycogen in animals represent reserve carbohydrates from which glucose can be rapidly mobilized to meet metabolic demands. Glucose fuels ATP synthesis and provides reducing power in the form of NADPH. When oxidized, glucose is completely converted to carbon dioxide (CO2) and water, releasing energy without generating toxic metabolic waste. From a nutritional standpoint, monosaccharides provide approximately 3.74 kcal/g, disaccharides about 3.95 kcal/g, and starch around 4.18 kcal/g; conventionally, the energy yield of carbohydrates is typically approximated at 4 kcal/g.
Protein-sparing effect
An adequate intake of carbohydrates exerts a protein-sparing effect by reducing the need to use proteins as an energy source. The oxidation of amino acids for energy is metabolically inefficient and results in the production of nitrogen- and sulfur-containing waste products (mainly as ammonia or urea), which must be eliminated by the body. By supplying sufficient carbohydrates, dietary proteins can be preserved for their primary structural and functional roles.
Role in lipid metabolism
Carbohydrates are essential for normal lipid metabolism. As famously stated by Pasteur, “fats burn in the fire of carbohydrates”, highlighting the dependence of fatty acid oxidation on carbohydrate availability. In addition, when consumed in excess, carbohydrates can be converted into fatty acids and triglycerides, a process that occurs mainly in the liver.
Glucose-dependent tissues
Certain tissues rely almost exclusively on glucose for energy production. Specific regions of the central nervous system depend on glucose to maintain their structural and functional integrity. Red blood cells are entirely dependent on glucose, as they lack mitochondria and cannot oxidize fatty acids or utilize alternative fuels.
Structural role in nucleic acids
The monosaccharides ribose and deoxyribose are fundamental structural components of RNA and DNA, respectively. These sugars are integral parts of nucleotide molecules and form the backbone of nucleic acids, underscoring the essential role of carbohydrates in genetic information storage and transmission.
Detoxification processes
Carbohydrates participate in detoxification reactions, particularly in the liver. Glucuronic acid, synthesized from glucose, conjugates with endogenous compounds such as hormones and bilirubin, as well as with exogenous substances including drugs, chemical toxins, and bacterial toxins. This process increases their solubility and facilitates their elimination.
Cell recognition and signaling
Carbohydrates are frequently covalently linked to proteins and lipids, forming glycoproteins and glycolipids. Within cells, these carbohydrate chains influence molecular trafficking and metabolic fate. On the cell surface, they play a critical role in cell–cell recognition and communication, such as immune cell interactions, the homing of lymphocytes to their lymph nodes of origin, leukocyte adhesion to damaged blood vessels, and sperm–oocyte recognition during fertilization.
Structural functions
Some polysaccharides primarily serve structural roles. Cellulose, the most abundant polysaccharide in nature, is a major component of plant cell walls, while chitin provides mechanical support in the exoskeletons of arthropods. In addition, heteropolysaccharides play a fundamental role in extracellular organization across all biological kingdoms. In bacteria, the rigid cell wall contains a heteropolysaccharide composed of two alternating monosaccharide units, whereas in animals, diverse heteropolysaccharides form a complex extracellular matrix that provides mechanical support, protection, and spatial organization to cells, tissues, and organs.
Technological and sensory functions
Finally, carbohydrates contribute to the flavor, texture, and palatability of many foods. In food processing, they influence viscosity, sweetness, browning reactions, and overall sensory properties.
References
- Alberts B., Johnson A., Lewis J., Morgan D., Raff M., Roberts K., Walter P. Molecular biology of the cell. 7th Edition. Garland Science, Taylor & Francis Group, 2022.
- Belitz H.-D., Grosch W., Schieberle P. Food chemistry. 4th Edition. Springer, 2009.
- Bender D.A. Benders’ dictionary of nutrition and food technology. 8th Edition. Woodhead Publishing. Oxford, 2006.
- Guyton A.C., Hall J.E. Textbook of medical physiology. 14th Edition. Philadelphia: Elsevier, 2021.
- FAO/WHO Expert consultation. Carbohydrates in human nutrition. FAO Food and nutrition paper No. 66. Rome: food and agriculture organization of the United Nations; 1998.
- Holesh J.E., Aslam S., Martin A. Physiology, Carbohydrates. [Updated 2023 May 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459280/
- Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 8th Edition. W.H. Freeman and Company, 2021.
- Soderberg T. Organic chemistry with a biological emphasis. Volume I. Chemistry Publications. 2019.
- Solomons T.W.G., Fryhle C.B., Snyder S.A. Solomons’ organic chemistry. 12th Edition. John Wiley & Sons Incorporated, 2017.
- Stipanuk M.H., Caudill M.A. Biochemical, physiological, and molecular aspects of human nutrition. 4th Edition. St. Louis: Elsevier, 2018.