Glycolysis, also known as the glycolytic pathway, from the Greek words glykys (“sweet”) and lysis (“dissolution” or “breakdown”), is the sequence of enzymatic reactions that occur in the cytosol and, even in the absence of oxygen, convert glucose, a six-carbon sugar, into two molecules of pyruvate, a three-carbon compound. This process is accompanied by the production of two molecules of ATP and NADH.[1]
Glycolysis, which evolved before a significant accumulation of oxygen in the atmosphere, is the metabolic pathway with the highest carbon flux in most living cells and is present in nearly all organisms.[1]
Because it does not require oxygen, glycolysis played a crucial role during the first two billion years of life’s evolution, representing one of the earliest biological mechanisms for energy extraction from organic molecules. In addition, it supplies key intermediates for aerobic catabolism and numerous biosynthetic pathways.[2]
Note: glycolysis is also known as the Embden–Meyerhof–Parnas pathway, named after Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas, the researchers who elucidated the pathway in muscle tissue.[3]
Summary: Key Points
- Definition: a sequence of 10 cytosolic enzymatic reactions which, even in the absence of oxygen, convert glucose into pyruvate, with the simultaneous production of 2 molecules of ATP and 2 of NADH.
- Fates of pyruvate: in the presence of oxygen, pyruvate can enter the Krebs cycle, while in the absence of oxygen it can be converted via lactic or alcoholic fermentation into lactate and ethanol, respectively.
- Source of precursors: provides precursors for different metabolic pathways, such as those involved in glycogen synthesis, fatty acids or 2,3-bisphosphoglycerate production.
- Regulation: mainly mediated by three key enzymes (hexokinase, phosphofructokinase-1, and pyruvate kinase) through allosteric mechanisms, covalent modifications, and hormonal signals.
Contents
- History
- Importance of glycolysis
- Sequence of reactions
- Fate of NADH and pyruvate
- Glycolysis and ATP production
- Feeder pathways of glycolysis
- Regulation of glycolysis
- References
History
The development of biochemistry progressed in parallel with the study of glucose metabolism, particularly glycolysis, the first metabolic pathway to be fully elucidated.[4][5]
Although the pathway was not completely mapped until the 1940s, a key discovery was made in 1897, by chance. A year earlier, German chemist M. Hahn, while attempting to obtain and preserve cell-free protein extracts of yeast, had encountered difficulties with their stability. His colleague Hans Buchner, recalling a method used for preserving jams, suggested adding sucrose to the extract.[6]
Eduard Buchner, Hans’ brother, applied this idea and observed that the solution produced bubbles. He concluded that fermentation was occurring, an entirely unexpected finding. According to Pasteur’s assertion in 1860, fermentation was thought to be inseparably linked to living cells. Eduard’s experiments, however, demonstrated that it could also occur in their absence. These results disproved the vitalist dogma and marked a turning point in the emergence of modern biochemistry.[7]
For this work, Eduard Buchner was awarded the Nobel Prize in Chemistry in 1907, becoming the first of several scientists later honored for discoveries related to glycolysis. Subsequent studies with muscle extracts revealed that many of the reactions involved in lactic fermentation were identical to those in alcoholic fermentation, highlighting the fundamental unity of biochemistry.[8]
As noted, glycolysis was eventually fully elucidated during the first half of the 20th century, thanks to the contributions of Gerty and Carl Cori, Carl Neuberg, Arthur Harden, William Young, Jacob Parnas, Otto Warburg, Hans von Euler-Chelpin, Gustav Embden, and Otto Meyerhof. Warburg and von Euler-Chelpin clarified the pathway in yeast, while Embden and Meyerhof did so in muscle tissue during the 1930s.[3]
Importance of glycolysis
Glycolysis is essential for most living cells, both as a source of energy and as a provider of precursors for many other metabolic pathways. The rate of carbon flow through glycolysis, that is, the amount of glucose converted to pyruvate per unit of time, is regulated to satisfy these two fundamental cellular demands.
Although relatively inefficient energetically, glycolysis can proceed in the absence of oxygen, a condition under which life first evolved and one still encountered by many modern cells, both eukaryotic and prokaryotic. Several examples illustrate this adaptability.[9]
Anaerobic glycolysis
In animals, skeletal muscle exhibits activity-dependent anaerobiosis; it can function without oxygen for short periods. During intense exercise, when ATP demand exceeds oxygen delivery, muscles continue to operate anaerobically, though only temporarily.
The cornea of the eye provides another example, because it is poorly vascularized.[9]
Many microorganisms also thrive in low-oxygen environments such as deep water, soil, or skin pores. Some, like Clostridium perfringens, Clostridium tetani, and Clostridium botulinum, which cause gangrene, tetanus, and botulism, respectively, are obligate anaerobes that cannot survive in the presence of oxygen.[10]
Glycolysis in glucose-dependent cells
Glycolysis also sustains cells and tissues where glucose is the sole source of energy, including:
- red blood cells, which lack mitochondria,
- sperm cells;
- the brain (which can also utilize ketone bodies during glucose scarcity);
- the adrenal medulla.[11]
A similar pattern occurs in plants. Many aquatic plants and starch-storing tissues (e.g., potato tubers) rely on glucose as their main energy source.[12]
Note: some organisms, known as facultative anaerobes, can survive both in the presence and absence of oxygen, switching between aerobic and anaerobic metabolism. For instance, Mytilus species exhibit habitat-dependent anaerobiosis, comparable to that of muscle tissue.[13]
Aerobic glycolysis
Under aerobic conditions, in cells containing mitochondria, glycolysis represents the first stage of the metabolic pathway that leads to the complete oxidation of glucose to carbon dioxide (CO2) and water (H2O), producing ATP.[14]
Sources of building blocks
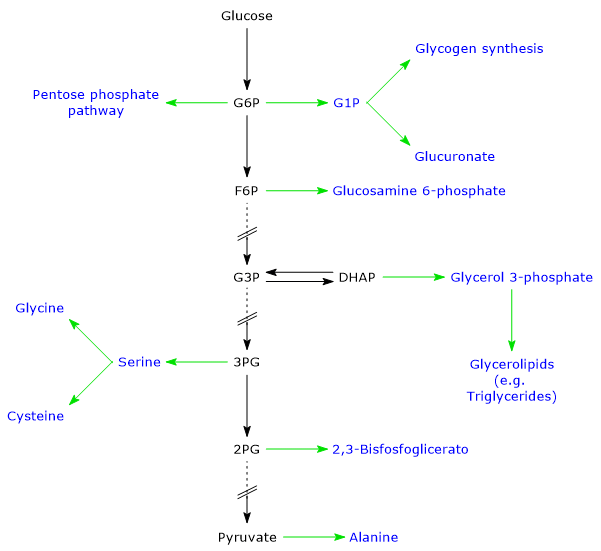
Several glycolytic intermediates, for example, glucose 6-phosphate (G6P), fructose 6-phosphate (F6P), and dihydroxyacetone phosphate (DHAP), serve as building blocks for other metabolic pathways. These include glycogen synthesis, as well as the biosynthesis of fatty acids, triglycerides, nucleotides, certain amino acids, and 2,3-bisphosphoglycerate (2,3-BPG).[15]
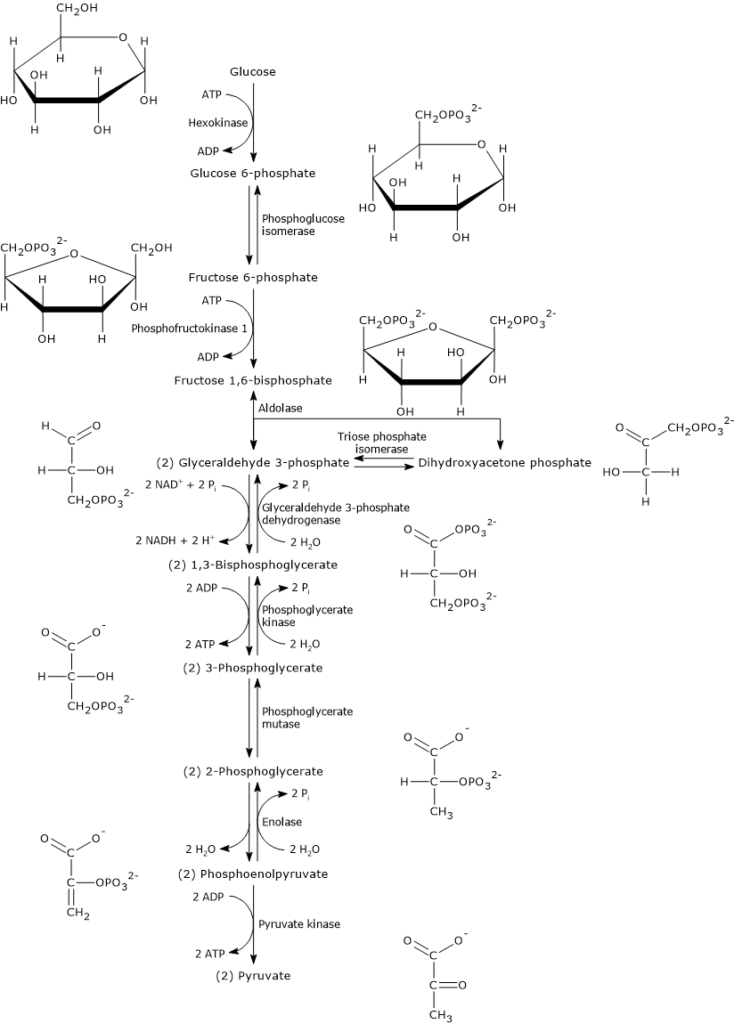
Sequence of reactions
The ten steps that constitute glycolysis can be divided into two phases.
The first, known as the preparatory phase, consists of five steps and begins with the conversion of glucose to fructose 1,6-bisphosphate (F1,6BP) through three enzymatic reactions: phosphorylation at C-6, isomerization, and a second phosphorylation at C-1, with the consumption of two ATP molecules.
Fructose 1,6-bisphosphate is then cleaved into two phosphorylated three-carbon compounds: glyceraldehyde 3-phosphate (G3P) and dihydroxyacetone phosphate. Finally, DHAP is isomerized into a second molecule of glyceraldehyde 3-phosphate. Thus, in the preparatory phase, one glucose molecule is split into two molecules of glyceraldehyde 3-phosphate, with a net consumption of two ATP molecules.
The second phase, the payoff phase, consists of the remaining five steps. In this phase, the two molecules of glyceraldehyde 3-phosphate are converted into two molecules of pyruvate, with the simultaneous production of four ATP molecules. Part of the energy stored in the chemical bonds of glucose is extracted and conserved in the form of ATP. In addition, reducing equivalents are captured and stored in the form of NADH, the reduced form of the coenzyme NAD+ (nicotinamide adenine dinucleotide). The metabolic fate of NADH depends on the cell type and whether conditions are aerobic or anaerobic.
Note: the glucose metabolized via the glycolytic pathway may originate either from extracellular glucose entering the cell through specific membrane transporters (derived from the bloodstream) or from intracellular glucose 6-phosphate produced by glycogenolysis.[11]
Reaction 1
In the first step of glycolysis, glucose is phosphorylated to glucose 6-phosphate at the expense of one ATP molecule.
Glucose + ATP → G6P + ADP
In most cells, this reaction is catalyzed by hexokinase (EC 2.7.1.1), an enzyme present in all organisms. In humans, four isoforms of hexokinase are expressed.[16] Both hexokinase and pyruvate kinase (EC 2.7.1.40), two of the key kinases of glycolysis, require a divalent metal ion such as magnesium (Mg2+) or manganese (Mn2+) for activity. Mg2+ binds to ATP, forming the MgATP2− complex, which is the true substrate of the enzyme rather than free ATP. The nucleophilic attack by the hydroxyl group (–OH) of glucose on the terminal (γ) phosphorus atom of ATP is facilitated by Mg2+, which stabilizes the negative charges on the phosphoryl groups of the nucleotide triphosphate.
The reaction involves the formation of a phosphoester bond between the phosphoryl group and the hydroxyl group at the C-6 position of glucose. This step is thermodynamically unfavorable and requires an input of energy, provided by ATP.[3]
Energetics and irreversibility
In the absence of ATP, the direct phosphorylation of glucose at C-6 by inorganic phosphate (Pi) is endergonic, with a standard Gibbs free energy change (ΔG°′) of +13.8 kJ/mol (+3.3 kcal/mol).
ATP hydrolysis to ADP and Pi has a ΔG°’ of –30.5 kJ/mol (–7.3 kcal/mol), an exergonic process.
The net ΔG°’ of the coupled reaction is –16.7 kJ/mol (–4.0 kcal/mol).
Under physiological conditions, the reaction is even more favorable, with a ΔG of ≈ –33.5 kJ/mol (–8.0 kcal/mol). Consequently, this step is considered essentially irreversible.[17]
Note: phosphorylation is a fundamental biochemical reaction catalyzed by kinases, a subclass of transferases. Kinases catalyze the transfer of the terminal phosphoryl group from a nucleoside triphosphate (usually ATP) to an acceptor nucleophile, forming a phosphoester bond. Specifically, hexokinase catalyzes the transfer of the γ-phosphoryl group of ATP to a variety of hexoses, including glucose, fructose, and mannose.[5]
| Step | Details |
|---|---|
| Reaction | Glucose + ATP → G6P + ADP |
| Enzyme | Hexokinase. In humans: four isoforms. |
| Cofactors | Mg2+ (as MgATP2−) or Mn2+ |
| ΔG°′ | –16.7 kJ/mol (net) (–4.0 kcal/mol) |
| ΔG | ≈ –33.5 kJ/mol (–8.0 kcal/mol) |
| Reversibility | Irreversible under physiological conditions |
Reaction 2
In the second step of glycolysis, G6P, an aldose, is isomerized to fructose 6-phosphate, a ketose.
G6P ⇄ F6P
This reaction is catalyzed by phosphoglucose isomerase (also known as phosphohexose isomerase or glucose phosphate isomerase; EC 5.3.1.9). The enzyme requires Mg2+ for activity. Glucose 6-phosphate and fructose 6-phosphate represent a case of functional group isomerism.
The ΔG°′ of the reaction is +1.7 kJ/mol (+0.4 kcal/mol), whereas the ΔG is ≈ –2.5 kJ/mol (–0.6 kcal/mol). These small values indicate that the reaction operates near equilibrium and is therefore readily reversible.
The reaction involves a shift of the carbonyl group from C-1 in the open-chain form of glucose 6-phosphate to C-2 in the open-chain form of fructose 6-phosphate.[3]
Mechanism and importance
The enzyme first opens the ring of glucose 6-phosphate, since both hexoses are mainly present in cyclic form in aqueous solution.
It then catalyzes the isomerization of the open-chain intermediate, shifting the carbonyl group from C-1 to C-2.
Finally, it promotes ring closure, forming fructose 6-phosphate, which adopts a five-membered furanose structure.[5]
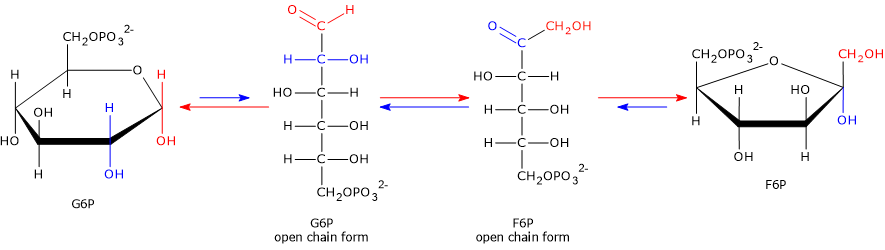
This isomerization is crucial because it prepares the substrate for subsequent reactions.
- In the third step, phosphorylation occurs at the C-1 position, which requires a hydroxyl group rather than a carbonyl group.
- In the fourth step, cleavage of the bond between C-3 and C-4 is facilitated by the presence of a carbonyl group at C-2, generated during this isomerization.[11]
| Step | Details |
|---|---|
| Reaction | G6P ⇄ F6P |
| Enzyme | Phosphoglucose isomerase |
| Cofactors | Mg2+ |
| ΔG°′ | +1.7 kJ/mol (+0.4 kcal/mol) |
| ΔG | ≈ –2.5 kJ/mol (–0.6 kcal/mol) |
| Reversibility | Reversible (near equilibrium) |
Reaction 3
In the third step of glycolysis, F6P undergoes a second phosphorylation. Phosphofructokinase-1 (PFK-1; EC 2.7.1.11) catalyzes the phosphorylation of fructose 6-phosphate at the C-1 position to yield fructose 1,6-bisphosphate, at the expense of one ATP molecule.
F6P + ATP → F1,6BP + ADP
PFK-1 is named to distinguish it from phosphofructokinase-2 (PFK-2; EC 2.7.1.105), which catalyzes the formation of fructose 2,6-bisphosphate (F2,6BP) from fructose 6-phosphate.[1]
Like the hexokinase-catalyzed reaction, this ATP-dependent phosphorylation step is essentially irreversible. Its irreversibility results from coupling to ATP hydrolysis, which provides the necessary free energy. Phosphorylation of fructose 6-phosphate by inorganic phosphate alone is endergonic, with a ΔG°′ of +16.3 kJ/mol (+3.9 kcal/mol). When coupled to ATP hydrolysis, however, the overall reaction becomes exergonic, with a ΔG°′ of –14.2 kJ/mol (–3.4 kcal/mol) and a cellular ΔG of ≈ –22.2 kJ/mol (–5.3 kcal/mol).[3]
While hexokinase functions primarily to trap glucose inside the cell, PFK-1 commits glucose-derived carbon to glycolysis rather than directing it toward glycogen synthesis or other biosynthetic pathways. Unlike glucose 6-phosphate, fructose 1,6-bisphosphate cannot readily enter alternative metabolic routes, making this the first committed step of glycolysis.
Committed steps are typically catalyzed by allosterically regulated enzymes, preventing the unnecessary accumulation of intermediates and end products. PFK-1 exemplifies this regulation, as it is subject to activation and inhibition by metabolites that reflect both the cell’s energy status and hormonal signals.[5]
| Step | Details |
|---|---|
| Reaction | F6P + ATP → F1,6BP + ADP |
| Enzyme | Phosphofructokinase-1 (PFK-1) |
| Cofactors | Mg2+ (as MgATP2–) |
| ΔG°′ | –14.2 kJ/mol (–3.4 kcal/mol) |
| ΔG | ≈ –22.2 kJ/mol (–5.3 kcal/mol) |
| Reversibility | Irreversible (first committed step of glycolysis) |
PPi dependent phosphofructokinase
In some protists and bacteria, and possibly in all plants, a variant phosphofructokinase uses pyrophosphate (PPi) instead of ATP as the phosphoryl donor. This alternative reaction has a ΔG°′ of –12.1 kJ/mol (–2.9 kcal/mol).[18]
F6P + PPi → F1,6BP + Pi
Reaction 4
In the fourth step of glycolysis, fructose 1,6-bisphosphate aldolase (commonly known as aldolase; EC 4.1.2.13) catalyzes the reversible cleavage of F1,6BP into two triose phosphates: glyceraldehyde 3-phosphate (an aldose) and dihydroxyacetone phosphate (a ketose). The enzyme cleaves the carbon–carbon bond between C-3 and C-4 of the fructose backbone.[1]
F1,6-BP ⇄ DHAP + G3P
From this step onward, all glycolytic intermediates are three-carbon compounds, whereas the preceding intermediates were six-carbon sugars.[11]
Note on nomenclature
The name “aldolase” derives from the reverse reaction, i.e., the condensation of an aldehyde and a ketone to form a β-hydroxyketone (an aldol), in a process known as aldol condensation.
Energetics and reversibility
The ΔG°′ of the cleavage reaction is +23.8 kJ/mol (+5.7 kcal/mol), with an equilibrium constant (Keq) of approximately 10-4 M. These values indicate that under standard conditions the reaction would not favor the forward (cleavage) direction. However, in the cellular context, the products are rapidly consumed in subsequent reactions, maintaining their concentrations at low levels. As a result, the actual free energy change is about –1.3 kJ/mol (–0.3 kcal/mol), showing that the reaction is near equilibrium and easily reversible.[5]
| Step | Details |
|---|---|
| Reaction | F1,6 ⇄ DHAP + G3P |
| Enzyme | Aldolase (Fructose 1,6-bisphosphate aldolase) |
| ΔG°′ | +23.8 kJ/mol (+5.7 kcal/mol) |
| ΔG | ≈ –1.3 kJ/mol (–0.3 kcal/mol) |
| Reversibility | Reaction near equilibrium; readily reversible |
| Note | The enzyme name originates from the reverse aldol condensation reaction |
Reaction 5
Of the two products generated in the previous step, glyceraldehyde 3-phosphate directly enters the second phase of glycolysis. In contrast, DHAP is not part of the main glycolytic route and must first be isomerized into glyceraldehyde 3-phosphate. This reversible reaction is catalyzed by triose phosphate isomerase (EC 5.3.1.1).
DHAP ⇄ G3P
Triose phosphate isomerase catalyzes a keto–enol tautomerization involving the transfer of a hydrogen atom from C-1 to C-2 of DHAP. As a consequence of this isomerization, the carbon atoms C-1, C-2, and C-3 of glucose become chemically indistinguishable from C-6, C-5, and C-4, respectively. The net result of reactions 4 and 5 is therefore the production of two molecules of glyceraldehyde 3-phosphate: one formed directly in reaction 4, and the other via isomerization.[3]
The ΔG°′ of this step is +7.5 kJ/mol (+1.8 kcal/mol), while the actual ΔG under cellular conditions is about +2.5 kJ/mol (+0.6 kcal/mol). At equilibrium, ≈ 96% of the triose phosphate pool exists as DHAP. Nonetheless, the reaction proceeds efficiently toward G3P because this intermediate is rapidly consumed in the following glycolytic step, driving the reaction forward.[1]
One notable feature of triose phosphate isomerase is its extraordinary catalytic efficiency. The enzyme is considered kinetically “perfect” because it accelerates the reaction rate by a factor of 1010 compared to a simple catalyst (e.g., acetate ion). Its catalytic efficiency, expressed as the Kcat/KM ratio, is about 2 x 108 M–1 s–1, approaching the diffusion-controlled limit. Thus, the rate-limiting factor is not the chemistry of the reaction itself but the rate at which substrate and enzyme encounter each other.[19]
Energetics and metabolic relevance
From a thermodynamic perspective, reactions 4 and 5 together are unfavorable, with a combined ΔG°′ of +31.3 kJ/mol (+7.5 kcal/mol). However, when the hydrolysis of two ATP molecules in earlier steps is taken into account, the overall ΔG°′ for the preparatory phase (steps 1–5) is only +2.1 kJ/mol (+0.5 kcal/mol). The corresponding Keq is ≈ 0.43. Importantly, under physiological conditions, the actual ΔG is strongly negative (≈ –56.8 kJ/mol; –13.6 kcal/mol), ensuring the pathway proceeds forward.[17]
Beyond glycolysis, DHAP can also serve as a precursor in lipid metabolism. It can be reduced to glycerol 3-phosphate by cytosolic glycerol 3-phosphate dehydrogenase (EC 1.1.1.8)
DHAP + NADH + H+ ⇄ Glycerol 3-phosphate + NAD+
This reaction links carbohydrate metabolism to lipid biosynthesis, as glycerol 3-phosphate is an essential precursor for triacylglycerols and phospholipids. In adipose tissue and the small intestine, this pathway represents a major source of glycerol 3-phosphate.[11]
| Step | Details |
|---|---|
| Reaction | DHAP ⇄ G3P |
| Enzyme | Triose phosphate isomerase |
| ΔG°′ | +7.5 kJ/mol (+1.8 kcal/mol) |
| ΔG | ≈ +2.5 kJ/mol (+0.6 kcal/mol), but pulled forward by rapid G3P utilization |
| Equilibrium | ≈ 96% DHAP at equilibrium |
| Catalytic efficiency | Kinetically “perfect”: Kcat/KM ≈ 2 x 108 M–1s–1 |
| Additional role | DHAP can be reduced to glycerol 3-phosphate, linking glycolysis to lipid metabolism |
Reaction 6
In the sixth step of glycolysis, and the first of the payoff phase, glyceraldehyde 3-phosphate dehydrogenase (EC 1.2.1.12) catalyzes the oxidation of G3P to 1,3-bisphosphoglycerate (1,3-BPG), coupled with the reduction of NAD+ to NADH.[3]
G3P + NAD+ + Pi ⇄ 1,3-BPG + NADH + H+
This is the first of two glycolytic reactions that conserve the chemical energy required for the subsequent synthesis of ATP.
Mechanism and energetics
The reaction consists of two tightly coupled processes.
- Oxidation: the aldehyde group of G3P is oxidized to a carboxylic acid, with NAD+ acting as the oxidizing agent. This process is strongly exergonic (ΔG°′ = –43.1 kJ/mol, −10.3 kcal/mol).
- Phosphorylation: a high-energy acyl phosphate bond is formed between the new carboxyl group and inorganic phosphate, yielding 1,3-BPG. This step is highly endergonic (ΔG°′ = +49.3 kJ/mol, +11.8 kcal/mol).[11]
Although each process alone would be thermodynamically unbalanced, their tight coupling allows the energy released from oxidation to drive acyl phosphate formation. The overall reaction has a ΔG°′ of +6.3 kJ/mol (+1.5 kcal/mol) and a cellular ΔG of ≈ +2.5 kJ/mol (+0.6 kcal/mol), both slightly endergonic.[17]
Thus, free energy is conserved in the form of the high-energy acyl phosphate bond of 1,3-BPG, rather than being lost as heat.[20]
| Aspect | Details |
|---|---|
| Reaction | G3P + NAD+ + Pi ⇄ 1,3-BPG + NADH + H+ |
| Enzyme | Glyceraldehyde 3-phosphate dehydrogenase |
| Cofactor | NAD+ (reduced to NADH) |
| ΔG°′ | +6.3 kJ/mol (+1.5 kcal/mol) |
| ΔG | ≈ +2.5 kJ/mol (+0.6 kcal/mol) |
| Importance | First energy-conserving step; formation of high-energy acyl phosphate bond |
Note on NAD+ reduction
The reversible reduction of NAD⁺ involves the transfer of two hydrogen atoms from the substrate, the aldehyde group of glyceraldehyde 3-phosphate undergoing oxidation: one as a hydride ion (2 e− + H+) to the nicotinamide ring, and the other as a free proton released into solution.[5]
Half-reactions:
NAD+ + 2 e– + 2 H+ → NADH + H+
NADP+ + 2 e– + 2 H+ → NADPH + H+
Reaction 7
In the seventh step of glycolysis, phosphoglycerate kinase (EC 2.7.2.3) catalyzes the transfer of a high-energy phosphoryl group from the acyl phosphate of 1,3-BPG to ADP, forming ATP and 3-phosphoglycerate (3-PG).[1]
1,3-BPG + ADP ⇄ 3-PG + ATP
The reaction has a ΔG°′ of −18.5 kJ/mol (−4.4 kcal/mol), making it exergonic, although under cellular conditions the free energy change is ≈ +1.3 kJ/mol (+0.3 kcal/mol).[17]
Energetics and reversibility
The high phosphoryl-transfer potential of the acyl phosphate group is harnessed to phosphorylate ADP in a process known as substrate-level phosphorylation. In other words, part of the energy released during the oxidation of glyceraldehyde 3-phosphate in the previous step is conserved in the synthesis of ATP.[5]
This is the first step in glycolysis where the chemical energy of glucose is captured as ATP. Since aldolase and triose phosphate isomerase (steps 4 and 5) yield two molecules of glyceraldehyde 3-phosphate per glucose, this step generates two ATP molecules, thereby offsetting the ATP investment made earlier (steps 1 and 3).
The enzyme name refers to the reverse reaction, i.e., the phosphorylation of 3-phosphoglycerate to form 1,3-bisphosphoglycerate using ATP. Like all enzymes, phosphoglycerate kinase can catalyze the reaction in both directions. The reverse reaction occurs in gluconeogenesis and in photosynthetic CO2 fixation.[21]
| Step | Details |
|---|---|
| Reaction | 1,3-BPG + ADP ⇄ 3-PG + ATP |
| Enzyme | Phosphoglycerate kinase |
| ΔG°′ | −18.5 kJ/mol (−4.4 kcal/mol) |
| ΔG | ≈ +1.3 kJ/mol (+0.3 kcal/mol) |
| Process | Substrate-level phosphorylation; ATP generation from the high-energy acyl phosphate of 1,3-BPG |
| ATP Yield | 2 ATP per glucose (since 2 × G3P enter the payoff phase) |
| Importance | First ATP-generating step in glycolysis; compensates the ATP invested in the preparatory phase |
| Reversibility | Reversible reaction; reverse direction occurs in gluconeogenesis and photosynthesis |
Coupling with reaction 6
Reactions 6 and 7 together constitute an energy-coupling system, with 1,3-BPG as the common intermediate.
While the synthesis of 1,3-BPG is endergonic (ΔG°′ = +6.3 kJ/mol; +1.5 kcal/mol), the subsequent phosphoglycerate kinase reaction is strongly exergonic (ΔG°′ = −18.5 kJ/mol, −4.4 kcal/mol). The net ΔG°′ is −12.2 kJ/mol (−2.9 kcal/mol), sufficient to drive not only the preceding dehydrogenase step, but also the aldolase and triose phosphate isomerase reactions.
G3P + ADP + Pi + NAD+ ⇄ 3-PG + ATP + NADH + H+
The phosphoryl group of 3-phosphoglycerate has a relatively low transfer potential (ΔG°′ ≈ −12.5 kJ/mol; −3 kcal/mol), insufficient to directly drive ATP synthesis. Therefore, in the next steps of glycolysis, 3-phosphoglycerate is converted into phosphoenolpyruvate (PEP), a compound with much higher phosphoryl-transfer potential.[3]
Reaction 8
In the eighth step of glycolysis, 3-PG is converted into 2-phosphoglycerate (2-PG) in a reversible reaction catalyzed by phosphoglycerate mutase (EC 5.4.2.1). This reaction requires Mg2+ and has a very small free energy change, with ΔG°′ = +4.4 kJ/mol (+1.1 kcal/mol) and ΔG ≈ +0.8 kJ/mol (+0.2 kcal/mol).[17]
Phosphoglycerate mutase belongs to the class of mutases, enzymes that catalyze intramolecular group transfers. In this case, it catalyzes the transfer of a phosphoryl group from the C-3 to the C-2 position of 3-PG. Mutases are a subclass of isomerases.[22]
3-PG ⇄ 2-PG
Mechanism and significance
The mechanism of this reaction varies among organisms. For example, in yeast and rabbit muscle, the reaction proceeds in two steps and involves the formation of phosphoenzyme intermediates.
In the first step, a phosphoryl group bound to a histidine residue in the enzyme’s active site is transferred to the hydroxyl group at C-2 of 3-PG, generating 2,3-bisphosphoglycerate.
In the second step, the enzyme acts as a phosphatase, converting 2,3-BPG into 2-phosphoglycerate, while the phosphoryl group at C-3 is transferred back to the histidine residue, regenerating the phosphorylated enzyme (enzyme–His–phosphate).
Schematically:
Enzyme-His-phosphate + 3-PG ⇄ Enzyme-His + 2,3-BPG
Enzyme-His + 2,3-BPG ⇄ Enzyme-His-phosphate + 2-PG
It is important to note that the phosphoryl group in 2-phosphoglycerate is not the same as the one originally present in 3-phosphoglycerate.[5]
| Step | Details |
|---|---|
| Reaction | 3-PG ⇄ 2-PG |
| Enzyme | Phosphoglycerate mutase |
| Cofactor | Mg2+; requires 2,3-bisphosphoglycerate for activation |
| ΔG°′ | +4.4 kJ/mol (+1.1 kcal/mol) |
| ΔG | ≈ +0.8 kJ/mol (+0.2 kcal/mol) |
| Mechanism | Proceeds via a phosphoenzyme intermediate and transient formation of 2,3-bisphosphoglycerate |
| Importance | Rearranges the phosphoryl group to prepare for the high-energy intermediate phosphoenolpyruvate |
2,3-BPG and the Rapoport-Luebering shunt
Approximately once every 100 catalytic cycles, 2,3-BPG dissociates from the enzyme, leaving it unphosphorylated and inactive. The enzyme can be reactivated by binding a new molecule of 2,3-BPG, which must therefore be present in the cytosol to maintain optimal activity. Small but sufficient amounts of 2,3-BPG are found in most cells, with the notable exception of red blood cells, where it is synthesized through the Rapoport−Luebering shunt. In erythrocytes, 2,3-BPG also acts as an allosteric inhibitor of hemoglobin, lowering its affinity for oxygen.[23][24]
Note: 3-phosphoglycerate also serves as a precursor for the biosynthesis of serine, from which glycine and cysteine are derived. The first step in serine biosynthesis is catalyzed by phosphoglycerate dehydrogenase (EC 1.1.1.95), which oxidizes 3-phosphoglycerate to 3-phosphohydroxypyruvate while reducing NAD+ to NADH. This step is rate-limiting, as serine acts as a feedback inhibitor of the enzyme.[25]
Reaction 9
In the ninth step of the glycolytic pathway, 2-PG is dehydrated to form phosphoenolpyruvate, an enol, in a reversible reaction catalyzed by enolase (EC 4.2.1.11).
2-PG ⇄ PEP + H2O
This reaction requires Mg2+, which stabilizes the enolic intermediate formed during the process.[11]
The ΔG°’ of the reaction is +7.5 kJ/mol (+1.8 kcal/mol), while ΔG = –3.3 kJ/mol (–0.8 kcal/mol), indicating that the reaction proceeds readily in vivo.[17]
Phosphoryl transfer potential and tautomerization
Like 1,3-bisphosphoglycerate, phosphoenolpyruvate possesses a phosphoryl group with a very high transfer potential, sufficient to allow ATP synthesis.
But why is the free energy of hydrolysis of PEP so high?
Although 2-PG and PEP contain nearly the same total amount of metabolic energy relative to complete oxidation to CO2, H2O and Pi, the dehydration of 2-PG redistributes this energy, leading to very different standard free energies of hydrolysis:
- –17.6 kJ/mol (–4.2 kcal/mol) for 2-phosphoglycerate (a phosphoric ester);
- –61.9 kJ/mol (–14.8 kcal/mol) for phosphoenolpyruvate (an enol phosphate).
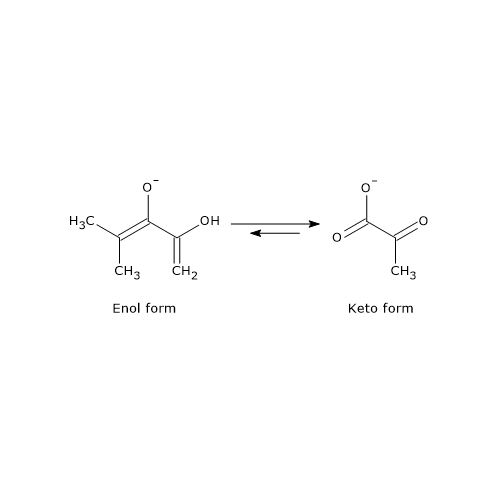
This difference arises because the phosphoryl group of PEP locks the molecule in its unstable enol form. In the final step of glycolysis, when PEP donates its phosphoryl group to ADP, ATP and the enol form of pyruvate are generated. The enol form of pyruvate is unstable and rapidly tautomerizes nonenzymatically into the more stable keto form, which predominates at physiological pH (≈7).
Thus, the extraordinarily high phosphoryl-transfer potential of PEP is due primarily to the subsequent enol–keto tautomerization of pyruvate, which provides a strong thermodynamic driving force.[5][26]
| Step | Details |
|---|---|
| Reaction | 2-PG ⇄ PEP + H2O |
| Enzyme | Enolase |
| Cofactor | Mg2+ (stabilizes the enolic intermediate) |
| ΔG°′ | +7.5 kJ/mol (+1.8 kcal/mol) |
| ΔG | ≈ –3.3 kJ/mol (–0.8 kcal/mol) |
| Phosphoryl transfer potential | PEP: –61.9 kJ/mol (–14.8 kcal/mol) vs 2-PG: –17.6 kJ/mol (–4.2 kcal/mol) |
| Importance | PEP stores energy in an unstable enol form; tautomerization of pyruvate (enol → keto) drives high phosphoryl-transfer potential |
Reaction 10
In the final step of glycolysis, pyruvate kinase catalyzes the transfer of a phosphoryl group from PEP to ADP, producing pyruvate and ATP. This is the second example of substrate-level phosphorylation in the pathway.
PEP + ADP → Pyruvate + ATP
Pyruvate kinase is a tetrameric enzyme and, like PFK-1, it is highly regulated. It contains binding sites for several allosteric effectors. In vertebrates, at least three isozymes exist: the M isozyme (predominant in muscle and brain) and the L isozyme (predominant in liver) are the best characterized. Although they share many properties, they differ in their regulation by hormones such as glucagon, epinephrine, and insulin.
Enzyme activity is also stimulated by potassium ions (K⁺) and other monovalent cations.[1][11][27]
The reaction is essentially irreversible, with a ΔG°′ of –31.4 kJ/mol (–7.5 kcal/mol) and a cellular ΔG of ≈ –16.7 kJ/mol (–4.0 kcal/mol). This strong thermodynamic favorability is largely due to the spontaneous tautomerization of pyruvate from its unstable enol form to its stable keto form.[5]
Energetics and reversibility
Of the –61.9 kJ/mol (–14.8 kcal/mol) released from the hydrolysis of the phosphoryl group of PEP, nearly half is conserved in the formation of the phosphoanhydride bond between ADP and Pi, whose ΔG°’ is –30.5 kJ/mol (–7.3 kcal/mol). The remaining energy (–31.4 kJ/mol or –7.5 kcal/mol) serves as the driving force that pushes the reaction toward ATP production.[17]
While the reaction catalyzed by phosphoglycerate kinase (step 7) restores the ATP “debt” from the preparatory phase, the pyruvate kinase reaction yields a net gain of two ATP molecules per glucose.
| Step | Details |
|---|---|
| Reaction | PEP + ADP → Pyruvate + ATP |
| Enzyme | Pyruvate kinase |
| Cofactors / Activators | K+ and other monovalent cations; allosteric regulation (isozyme-specific) |
| ΔG°′ | –31.4 kJ/mol (–7.5 kcal/mol) |
| ΔG | ≈ –16.7 kJ/mol (–4.0 kcal/mol) |
| Isozymes | M isozyme (muscle, brain); L isozyme (liver); regulated differently by hormones |
| Importance | Second substrate-level phosphorylation; net gain of 2 ATP per glucose; irreversibility ensured by pyruvate tautomerization |
Fate of NADH and pyruvate
Glycolysis yields 2 NADH, 2 ATP, and 2 pyruvate molecules per molecule of glucose.[1]
For glycolysis to continue, NADH must be reoxidized to NAD+. This coenzyme, derived from vitamin B3 (niacin), is present in the cytosol at a concentration of ≤ 10−5M, far lower than the amount of glucose metabolized within a few minutes, and therefore must be continuously regenerated. The regeneration of NAD+ from NADH constitutes the final step of the glycolytic pathway and can proceed through either aerobic or anaerobic routes, both of which involve pyruvate. These pathways are essential for maintaining the cell’s redox balance.[28]
Pyruvate itself is a versatile metabolite that can participate in several metabolic fates, both anabolic and catabolic, depending on the cell type, the energy status of the cell, and the availability of oxygen.[29]
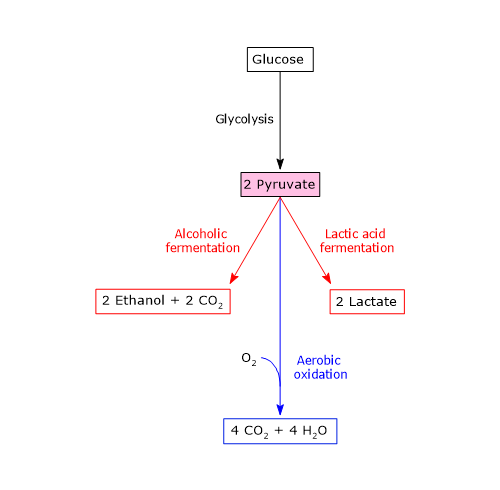
With the exception of certain bacterial variations, exploited, for example, in the food industry for the production of specific fermented products such as cheese, pyruvate typically follows one of three catabolic routes:
- reduction to lactate, via lactic acid fermentation;
- reduction to ethanol, via alcoholic fermentation;
- aerobic oxidation.
These pathways allow glycolysis to proceed under both anaerobic and aerobic conditions. Thus, the catabolic fate of glucose carbon skeletons depends on cell type, energetic state, and oxygen availability.[11]
Lactic acid fermentation
In animals, aside from a few exceptions, and in many microorganisms, when oxygen availability is insufficient to meet the cell’s energy demands, or when the cell lacks mitochondria, the pyruvate generated by glycolysis is reduced to lactate in the cytosol. This reaction is catalyzed by lactate dehydrogenase (EC 1.1.1.27).[11]
Pyruvate + NADH + H+ ⇄ Lactate + NAD+
Here, pyruvate acts as the electron acceptor and is reduced to lactate, while NAD+ is regenerated. The equilibrium strongly favors lactate formation, as indicated by the ΔG°′ of –25.1 kJ/mol (–6.0 kcal/mol).[30]
The overall equation for the conversion of glucose to lactate, known as lactic acid fermentation, is:
Glucose + 2 Pi + 2 ADP → 2 Lactate + 2 ATP + 2 H2O
Discovered by Louis Pasteur, who famously described it as “la vie sans l’air”, fermentation is a metabolic process that:
- extracts energy from glucose, conserving it as ATP;
- does not consume oxygen;
- does not alter the overall NAD+/NADH ratio.[31]
Although NAD+ and NADH do not appear in the overall reaction, both are essential intermediates. Thus, there is no net oxidation or reduction: the hydrogen-to-carbon ratio remains unchanged in the conversion of glucose (C6H12O6) to lactate (C3H6O3).[1]
However, from an energetic standpoint, fermentation captures only a small fraction of the total chemical energy stored in glucose.
Fate of lactic acid
In humans, much of the lactate produced is directed into the Cori cycle, where it is transported to the liver and used for glucose synthesis via gluconeogenesis. This process shifts part of the metabolic load from extrahepatic tissues, such as skeletal muscle during intense exercise (e.g., a 200-meter sprint, when glycolysis can increase up to 2,000-fold almost instantaneously), to the liver.[32]
Unlike skeletal muscle, which releases lactate into venous blood, cardiac muscle can take up and utilize lactate an energy substrate. This is due to its strictly aerobic metabolism and to the properties of its tissue-specific isozyme of lactate dehydrogenase, LDH-4. Consequently, a portion of the lactate released by active skeletal muscle is directly consumed by the heart.[33]
Note: in microorganisms, lactic acid fermentation produces lactate that contributes to the flavor and aroma of foods such as sauerkraut (fermented cabbage) and soured milk.[34]
Alcoholic fermentation
In microorganisms such as brewer’s and baker’s yeast, in certain plant tissues, and in some invertebrates and protists, pyruvate may be converted to ethanol under hypoxic or anaerobic conditions, with the concomitant release of CO2. This occurs in two enzymatic steps.[32]
In the first step, pyruvate undergoes non-oxidative decarboxylation to form acetaldehyde. This essentially irreversible reaction is catalyzed by pyruvate decarboxylase (EC 4.1.1.1), which requires Mg2+ and thiamine pyrophosphate, a coenzyme derived from thiamine (vitamin B1). Pyruvate decarboxylase is absent in vertebrates and organisms that carry out lactic acid fermentation.
In the second step, acetaldehyde is reduced to ethanol in a reaction catalyzed by alcohol dehydrogenase (EC 1.1.1.1). This enzyme contains a bound zinc ion at its active site. NADH provides the reducing equivalents and is oxidized to NAD⁺. At physiological pH, the equilibrium strongly favors ethanol formation.
The overall process, known as alcoholic fermentation, is summarized by the equation:
Glucose + 2 Pi + 2 ADP → 2 Ethanol + 2 CO2 + 2 ATP + 2 H2O
As in lactic acid fermentation, no net oxidation–reduction occurs.[35]
Alcoholic fermentation is the biochemical basis for the production of beer and wine. The CO2 released by brewer’s yeast generates the bubbles characteristic of beer, champagne, and sparkling wines, while the CO2 produced by baker’s yeast causes dough to rise.[36]
Fate of pyruvate and NADH under aerobic conditions
In cells that contain mitochondria and under aerobic conditions, the predominant situation in both multicellular organisms and many unicellular species, the oxidation of NADH and the catabolism of pyruvate proceed through distinct but interconnected pathways.
Within the mitochondrial matrix, pyruvate is first converted to acetyl-CoA by the pyruvate dehydrogenase complex, a large multienzyme complex. During this oxidative decarboxylation, pyruvate loses, as part of a carboxyl group, one carbon atom in the form of CO2, and the remaining two-carbon fragment is transferred to coenzyme A, forming acetyl-CoA.[37][38]
Pyruvate + NAD+ + CoA → Acetyl-CoA + CO2 + NADH + H+
The acetyl group of acetyl-CoA is then completely oxidized to CO2 via the citric acid cycle, with the concurrent production of NADH and FADH2. The pyruvate dehydrogenase complex thus constitutes a crucial metabolic link between glycolysis, which occurs in the cytosol, and the citric acid cycle, which takes place in the mitochondrial matrix.[32]
Electrons generated during glycolysis are transferred into the mitochondria indirectly: cytosolic NADH reduces specific shuttle intermediates, which are then reoxidized inside the mitochondrial matrix. In this way, NADH is effectively reoxidized to NAD+ in the cytosol, while the reduced intermediates deliver electrons to Complex I of the electron transport chain. From there, electrons are passed through the chain to oxygen, producing H2O. This electron transfer provides the free energy required for ATP synthesis via oxidative phosphorylation.
Electrons carried by NADH formed during the pyruvate dehydrogenase reaction and the citric acid cycle, as well as those derived from FADH2, follow a similar path toward oxidative phosphorylation.[39]
Note: FADH₂ does not transfer its reducing equivalents to Complex I but instead donates them to Complex II.[40]
Anabolic fates of pyruvate
Under anabolic conditions, the carbon skeleton of pyruvate can follow fates other than complete oxidation to CO2 or reduction to lactate. After its conversion to acetyl-CoA, for example, pyruvate may serve as a precursor for fatty acid biosynthesis or for the synthesis of the amino acid alanine.[41][42]
Glycolysis and ATP production
In the glycolytic pathway, one molecule of glucose is degraded into two molecules of pyruvate.
- During the preparatory phase, two molecules of ATP are consumed per molecule of glucose in the reactions catalyzed by hexokinase and PFK-1.
- During the payoff phase, four molecules of ATP are produced via substrate-level phosphorylation in the reactions catalyzed by phosphoglycerate kinase and pyruvate kinase.
Thus, the net gain is two ATP molecules per glucose molecule. In addition, the reaction catalyzed by glyceraldehyde 3-phosphate dehydrogenase produces two molecules of NADH per glucose.
The overall ΔG°′ of glycolysis is –85 kJ/mol (–20.3 kcal/mol). This value results from the difference between:
- the ΔG°′ of glucose conversion into two molecules of pyruvate (–146 kJ/mol; –34.9 kcal/mol), and
- the ΔG°′ required for ATP formation from ADP and Pi: 2 × 30.5 kJ/mol = 61 kJ/mol (2 × 7.3 kcal/mol = 14.6 kcal/mol).
The two reactions can be written as:
Glucose + 2 NAD+ → 2 Pyruvate + 2 NADH + 2 H+
2 ADP + 2 Pi → 2 ATP + 2 H2O
Summing these gives the overall glycolytic equation:
Glucose + 2 NAD+ + 2 ADP + 2 Pi → 2 Pyruvate + 2 NADH + 2 H+ + 2 ATP + 2 H2O
Under standard conditions, the fraction of energy released that is conserved in ATP is:
(61/146) x 100 = 41.8%.
Alternatively, the overall glycolytic equation can be derived by including all intermediates (ATP, NAD+, ADP, and Pi) as reactants and products:
Glucose + 2 ATP + 2 NAD+ + 4 ADP + 2 Pi → 2 Pyruvate + 2 ADP + 2 NADH + 2 H+ + 4 ATP + 2 H2O
By canceling common terms on both sides, this reduces to the standard overall equation shown above.[17]
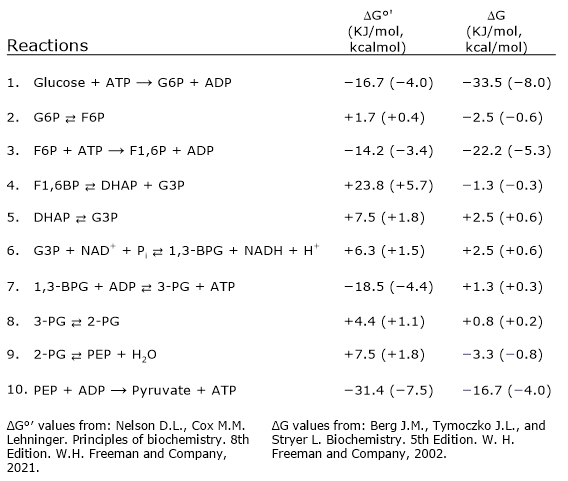
Free energy changes of glycolytic reactions
The direction and regulation of glycolysis depend on the free energy changes of its individual steps. The table below summarizes the standard and cellular ΔG values for each reaction, showing that only a few steps are highly exergonic and essentially irreversible under physiological conditions.[17]
A graphical version is shown below for quick reference.
| Step | Reaction | ΔG°′ (kJ/mol, kcal/mol) | ΔG (kJ/mol, kcal/mol) |
|---|---|---|---|
| 1 | Glu + ATP → G6P + ADP | –16.7 (–4.0) | –33.5 (–8.0) |
| 2 | G6P ⇄ F6P | +1.7 (+0.4) | –2.5 (–0.6) |
| 3 | F6P + ATP → F1,6BP + ADP | –14.2 (–3.4) | –22.2 (–5.3) |
| 4 | F1,6P ⇄ DHAP + G3P | +23.8 (+5.7) | –1.3 (–0.3) |
| 5 | DHAP ⇄ G3P | +7.5 (+1.8) | +2.5 (+0.6) |
| 6 | G3P + NAD+ + Pi ⇄ 1,3-BPG + NADH + H+ | +6.3 (+1.5) | +2.5 (+0.6) |
| 7 | 1,3-BPG + ADP ⇄ 3-PG + ATP | –18.5 (–4.4) | +1.3 (+0.3) |
| 8 | 3-PG ⇄ 2-PG | +4.4 (+1.1) | +0.8 (+0.2) |
| 9 | 2-PG ⇄ PEP + H2O | +7.5 (+1.8) | –3.3 (–0.8) |
| 10 | PEP + ADP → Pyr + ATP | –31.4 (–7.5) | –16.7 (–4.0) |
| ΔG°′ values from: Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 8th Edition. W.H. Freeman and Company, 2021. | ΔG values from: Berg J.M., Tymoczko J.L., Gregory J.G. Jr, Stryer L. Biochemistry. 9th Edition. W.H. Freeman and Company, 2019. | ||
ATP production under anaerobic conditions
Under anaerobic conditions, there is no additional ATP production beyond glycolysis, regardless of the metabolic fate of pyruvate, whether it is converted to lactate, ethanol, or other molecule.[43]
Therefore, glycolysis extracts only a small fraction of the chemical energy contained in a glucose molecule, which has a total energy yield of approximately 2,840 kJ/mol (679 kcal/mol) when fully oxidized to CO2 and H2O. In contrast, the conversion of glucose to two molecules of pyruvate releases only 146 kJ/mol, corresponding to ≈ 5% [(146/2,840) × 100] of the total available chemical energy. As a result, pyruvate retains most of the energy of the original hexose. Likewise, the four electrons carried by the NADH molecules produced during step 6 of glycolysis cannot contribute to ATP production under anaerobic conditions.[17]
In lactic acid fermentation, the ΔG°′ for the conversion of one molecule of glucose to two molecules of lactate is –183.6 kJ/mol (–43.9 kcal/mol). Of this free energy, ≈ 33.2% [(61/183.6) × 100] is stored in the two molecules of ATP formed. This is lower than the ≈41.8% efficiency of energy storage observed during glycolysis when glucose is converted to pyruvate.[44]
It should be noted that under physiological conditions, the free energy required to synthesize ATP from ADP and Pi is significantly higher than under standard conditions. Consequently, only about 50% of the energy released during glycolysis is actually stored in ATP in vivo.[1]
ATP production under aerobic conditions
Under aerobic conditions, cells with mitochondria can extract far more chemical energy from glucose and conserve it as ATP than under anaerobic conditions.[14]
The two molecules of NADH produced during glycolysis carry four reducing equivalents. When these electrons are transferred through the mitochondrial electron transport chain via oxidative phosphorylation, they yield approximately 2 to 3 ATP per electron pair. Consequently, the conversion of one molecule of glucose into two molecules of pyruvate can generate a total of 6 to 8 ATP: 2 ATP via substrate-level phosphorylation during glycolysis and 4 to 6 ATP through oxidative phosphorylation.[40]
Note: the exact number of ATP molecules generated from cytosolic NADH depends on the shuttle system used to transfer reducing equivalents into the mitochondria.
Furthermore, if we consider the complete aerobic oxidation of glucose through the coordinated action of glycolysis, the pyruvate dehydrogenase complex, the citric acid cycle, the electron transport chain, and oxidative phosphorylation, the ATP yield is much higher. According to Lehninger’s Principles of Biochemistry, the total ATP yield per glucose molecule ranges from 30 to 32. More recent estimates suggest a net production of approximately 29.85 ATP per glucose molecule, or 29.38 ATP if the ATP derived from GTP in the citric acid cycle is exported to the cytosol. In either case, aerobic metabolism yields roughly 15 times more ATP than anaerobic metabolism.[1]
Feeder pathways of glycolysis
Carbohydrates other than glucose, both simple and complex, can also be catabolized via glycolysis after their enzymatic conversion into one of the glycolytic intermediates.
Among the most important are:
- glycogen and starch, two major storage polysaccharides;
- disaccharides such as sucrose, maltose, lactose, and trehalose;
- monosaccharides including glucose, galactose, fructose, and the less common mannose.[45]
In the intestine, starch and disaccharides are hydrolyzed by digestive enzymes during carbohydrate digestion. The resulting monosaccharides, released directly or derived from the diet, are absorbed and transported into the venous circulation. From there, they reach the liver through the portal vein, which serves as the primary site for their metabolic processing.[46]
Glycogen and starch
For details on the phosphorolytic degradation of starch and endogenous glycogen, refer to the dedicated sections.
Fructose
Under physiological conditions, the liver removes much of the ingested fructose from the bloodstream before it can reach extrahepatic tissues.[47]
The hepatic pathway for converting fructose into glycolytic intermediates consists of several steps.[32]
In the first step, fructose is phosphorylated to fructose 1-phosphate (F1P) at the expense of one ATP molecule. This reaction, catalyzed by fructokinase (EC 2.7.1.4), requires Mg2+.
Fructose + ATP → F1P + ADP
As with glucose, phosphorylation traps fructose inside the cell.
In the second step, fructose 1-phosphate aldolase catalyzes an aldol cleavage of fructose 1-phosphate, yielding dihydroxyacetone phosphate and glyceraldehyde.
F1P → DHAP + Glyceraldehyde
Dihydroxyacetone phosphate is a direct glycolytic intermediate and, once converted to glyceraldehyde 3-phosphate, can continue through the pathway.
By contrast, glyceraldehyde is not a glycolytic intermediate and must be phosphorylated to glyceraldehyde 3-phosphate at the expense of another ATP molecule. This reaction, catalyzed by triose kinase (EC 2.7.1.28), also requires Mg2+.
Glyceraldehyde + ATP → G3P + ADP
Thus, in hepatocytes, one molecule of fructose is converted into two molecules of glyceraldehyde 3-phosphate, at the cost of two ATP molecules, just as with glucose.[48]
Fructose + 2 ATP → 2 G3P + 2 ADP
Fructose and hexokinase
In addition to the hepatic fructokinase pathway, extrahepatic tissues can also metabolize fructose through the action of hexokinase, albeit with a markedly lower affinity for fructose than for glucose.
In these tissues, such as skeletal muscle, kidney, and adipose tissue, fructokinase is absent. Instead, fructose enters glycolysis as fructose 6-phosphate, following its phosphorylation at C-6 catalyzed by hexokinase.
Fructose + ATP → F6P + ADP
However, the enzyme’s affinity for fructose is about 20 times lower than for glucose. Consequently, in hepatocytes, where glucose is abundant, or in skeletal muscle under anaerobic conditions, when glucose is the preferred fuel, only small amounts of fructose 6-phosphate are produced.[11][47]
In contrast, in adipose tissue, fructose levels can exceed those of glucose, and its phosphorylation by hexokinase is not significantly inhibited. Thus, in this tissue, the formation of fructose 6-phosphate represents the primary entry point of fructose into glycolysis.[49]
Metabolic implications
It is important to note that in the liver, fructose, being phosphorylated at C-1, enters glycolysis at the level of triose phosphates, downstream of the reaction catalyzed by PFK-1, the key regulatory enzyme of glycolytic flux.
Conversely, when fructose is phosphorylated at C-6, it enters glycolysis upstream of PFK-1.[9]
Sorbitol
Fructose represents the entry point into glycolysis for sorbitol, a sugar naturally present in many fruits and vegetables, and also widely used as a sweetener and stabilizer.
In the liver, sorbitol dehydrogenase (EC 1.1.1.14) catalyzes the oxidation of sorbitol to fructose in a reaction that requires zinc ions and NAD+ as a cofactor.[32] This enzyme also participates in the polyol pathway, which interconverts glucose, sorbitol, and fructose and plays an important role in tissues such as the liver, seminal vesicles, and retina.[48]
Sorbitol + NAD+ → Fructose + NADH + H+
Galactose
Galactose, primarily derived from the intestinal digestion of lactose, is metabolized in the liver via the Leloir pathway, which converts it into glucose 1-phosphate.[50]
The metabolic fate of glucose 1-phosphate depends on the energy status of the cell.
- Under conditions favoring glucose storage, glucose 1-phosphate is directed toward glycogen synthesis.
- Under conditions favoring glucose utilization as a fuel, glucose 1-phosphate is isomerized to glucose 6-phosphate in the reversible reaction catalyzed by phosphoglucomutase (EC 5.4.2.2):
G1P ⇄ G6P
Glucose 6-phosphate can then either enter glycolysis for energy production or be dephosphorylated by glucose 6-phosphatase (EC 3.1.3.9) to free glucose, which can be released into the bloodstream.[51][52]
Mannose
Mannose is found in various dietary polysaccharides, glycolipids, and glycoproteins. In the intestine, it is released from these macromolecules, absorbed, and then transported to the liver. There, it is phosphorylated at C-6 to form mannose 6-phosphate (M6P) in a reaction catalyzed by hexokinase.
Mannose + ATP → M6P + ADP
The resulting sugar phosphate is subsequently isomerized to fructose 6-phosphate by mannose 6-phosphate isomerase (EC 5.3.1.8).[53]
M6P ⇄ F6P
Regulation of glycolysis
The flux of carbon through the glycolytic pathway is tightly regulated in response to metabolic conditions, both intracellular and extracellular, in order to satisfy two major needs:
- the production of ATP;
- the supply of precursors for biosynthetic reactions.
In the liver, glycolysis and gluconeogenesis are reciprocally regulated to avoid a wasteful expenditure of energy: when one pathway is active, the other is inhibited. Evolutionarily, this control was achieved by selecting distinct enzymes to catalyze the essentially irreversible reactions of the two pathways. These enzymes are regulated independently, thereby avoiding the risk of a futile cycle (also known as a substrate cycle) that would result if both reactions occurred simultaneously at high rates. Such precise regulation would not be possible if a single enzyme catalyzed both directions of the reaction.[7]
The control of glycolysis primarily involves three enzymes: hexokinase, phosphofructokinase-1, and pyruvate kinase. Their activities are regulated through:
- allosteric modifications, which occur on a millisecond timescale and are rapidly reversible;
- covalent modifications (phosphorylation and dephosphorylation), which take place on a timescale of seconds;
- changes in enzyme concentration, due to altered rates of synthesis and/or degradation, which occur over hours.
Note: the main regulatory enzymes of gluconeogenesis are pyruvate carboxylase (EC 6.4.1.1) and fructose 1,6-bisphosphatase (FBPasi-1; EC 3.1.3.11).[1][11]
Hexokinase
In humans, hexokinase exists as four tissue-specific isozymes, hexokinase I, II, III, and IV, each of which is encoded by a distinct gene.[16]
Hexokinase I is the predominant isozyme in the brain. In skeletal muscle, both hexokinase I and II are expressed, contributing approximately 70–75% and 25–30% of total hexokinase activity, respectively.[9]
Hexokinase IV, also known as glucokinase (EC 2.7.1.2), is primarily expressed in hepatocytes and pancreatic β-cells, where it is the predominant isozyme. In the liver, glucokinase participates, together with glucose 6-phosphatase, in the substrate cycle between glucose and glucose 6-phosphate. Glucokinase differs from the other hexokinase isozymes in both its kinetic and regulatory properties.[54]
Note: isozymes (or isoenzymes) are distinct proteins that catalyze the same reaction but generally differ in kinetic and regulatory properties, subcellular localization, or cofactor requirements. They may coexist within the same organism, the same tissue, or even the same cell.[55]
Kinetic properties of hexokinase isozymes
The kinetic properties of hexokinase I, II, and III are broadly similar.
- Hexokinase I and II have Km values for glucose of 0.03 mM and 0.1 mM, respectively. These low Km values allow the enzymes to function efficiently at normal blood glucose concentrations (4–5 mM).
- By contrast, glucokinase has a high Km for glucose (≈ 10 mM), meaning it is active primarily when blood glucose levels are elevated, such as after a carbohydrate-rich meal.[9]
Regulation of the activity of hexokinases I-III
Hexokinases I–III are allosterically inhibited by glucose 6-phosphate, the product of their reaction.[16] This feedback mechanism prevents the accumulation of glucose 6-phosphate in the cytosol when glucose is not required for:
- ATP production;
- glycogen synthesis;
- the pentose phosphate pathway;
- other biosynthetic processes.[5]
At the same time, this regulation allows glucose to remain in the bloodstream, available to other tissues. For example, inhibition of phosphofructokinase-1 leads to the accumulation of fructose 6-phosphate, which, via the phosphoglucose isomerase reaction, increases glucose 6-phosphate levels. Consequently, inhibition of PFK-1 indirectly inhibits the activity of hexokinases I–III.[56]
Coordination of hexokinase and GLUT4 in skeletal muscle
In skeletal muscle, the activities of hexokinase I and II are tightly coordinated with those of GLUT4, a glucose transporter with a Km of ≈ 5 mM. Translocation of GLUT4 to the plasma membrane is stimulated by both insulin and physical activity. The combined action of GLUT4-mediated glucose uptake and cytosolic hexokinase activity maintains a balance between glucose entry and its phosphorylation.[9]
Since blood glucose concentrations typically range between 4 and 5 mM, GLUT4-mediated uptake increases intracellular glucose to levels sufficient to saturate, or nearly saturate, hexokinase, allowing the enzyme to operate at or near its Vmax.[57]
Hepatic glucokinase
Glucokinase differs in three key respects from hexokinases I–III and is particularly well-suited to the liver’s role in regulating blood glucose levels.[54][58]
Kinetic and functional properties
As previously mentioned, glucokinase has a Km for glucose of about 10 mM, much higher than that of hexokinases I–III and also higher than fasting blood glucose levels (4–5 mM). In the liver, where glucokinase is the predominant hexokinase isoenzyme, its role is to provide glucose 6-phosphate for the synthesis of glycogen and fatty acids. The activity of glucokinase is linked to that of GLUT2, the main glucose transporter in hepatocytes, which also has a high Km for glucose (approximately 10 mM).[16]
Hence, GLUT2 is highly active when blood glucose levels are elevated, rapidly equilibrating glucose concentrations between the cytosol of hepatocytes and the blood. Under such conditions, glucokinase is active and converts glucose into G6P. Due to its high Km, glucokinase activity continues to rise even as intracellular glucose concentrations reach or exceed 10 mM. Therefore, the rate of glucose uptake and phosphorylation is directly determined by blood glucose levels.[59]
Conversely, when glucose availability is low, its cytosolic concentration in hepatocytes is also low, much lower than the Km of glucokinase, which ensures that glucose produced through gluconeogenesis and/or glycogenolysis is not phosphorylated and can exit the cell.[60] A similar situation also occurs in pancreatic β-cells, where the GLUT2/glucokinase system aligns intracellular glucose 6-phosphate levels with blood glucose levels, allowing the cells to detect and respond to hyperglycemia.[61]
Regulation by glucokinase regulatory protein
Unlike hexokinases I–III, glucokinase is not inhibited by glucose 6-phosphate, that is, it is not subject to product inhibition, and continues to catalyze glucose phosphorylation even when glucose 6-phosphate accumulates. Glucokinase is inhibited by reversible binding to the glucokinase regulatory protein (GKRP), a liver-specific regulator. This inhibition involves the sequestration of glucokinase in the nucleus, separating it from other glycolytic enzymes.[60][62]
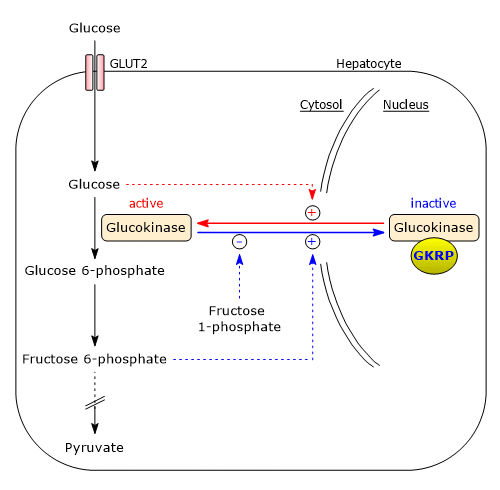
The binding between glucokinase and GKRP is strengthened by fructose 6-phosphate, whereas it is weakened by glucose and fructose 1-phosphate.
In the absence of glucose, glucokinase adopts its “super-open” conformation, which is capable of binding to GKRP. Rising cytosolic glucose concentrations trigger a concentration-dependent shift to the “closed” conformation, its active form, which cannot bind GKRP. As a result, glucokinase becomes active and is no longer inhibited.
Note that fructose 1-phosphate is present in the hepatocyte only when fructose is metabolized. Therefore, fructose relieves glucokinase inhibition by disrupting its interaction with GKRP.[63]
Physiological significance
After a carbohydrate-rich meal, blood glucose levels rise. Glucose enters hepatocytes via GLUT2 and then diffuses into the nucleus through nuclear pores. There, glucose promotes the transition of glucokinase to its closed, active conformation, which is no longer accessible to GKRP. This allows glucokinase to diffuse into the cytosol, where it phosphorylates glucose.
Conversely, when glucose concentration declines, such as during fasting when blood glucose levels may fall below 4 mM, glucose levels in hepatocytes also drop. Under these conditions, fructose 6-phosphate binds to GKRP, enhancing its affinity for glucokinase and resulting in strong inhibition of the enzyme. This mechanism ensures that, at low blood glucose levels, the liver does not compete with other organs, primarily the brain, for glucose.
Within the cell, fructose 6-phosphate is in equilibrium with glucose 6-phosphate due to the action of phosphoglucose isomerase. By binding to GKRP, fructose 6-phosphate contributes to the suppression of glucokinase activity, thereby preventing the accumulation of glycolytic intermediates.
To sum up, when blood glucose levels are within the normal range, glucose is phosphorylated primarily by hexokinases I–III. When blood glucose levels are high, glucokinase contributes to glucose phosphorylation as well.[9][64]
Regulation of phosphofructokinase-1 activity
Phosphofructokinase-1 is the key control point for carbon flow through glycolysis.[65]
In addition to substrate binding sites, the enzyme has several binding sites for allosteric effectors.
ATP, citrate, and hydrogen ions act as allosteric inhibitors of the enzyme, whereas AMP, Pi, and fructose 2,6-bisphosphate function as allosteric activators.[66][67][68]
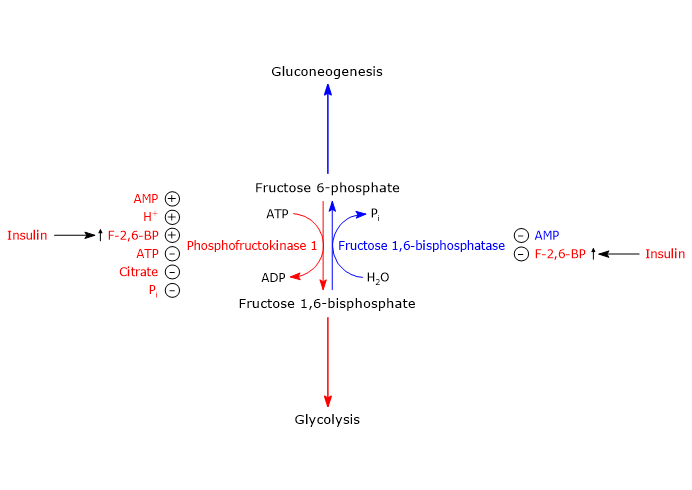
It is worth noting that ATP, an end product of glycolysis, is also a substrate of phosphofructokinase-1. The enzyme possesses two binding sites for the nucleotide: a low-affinity regulatory site and a high-affinity substrate site.[9]
What allosteric effectors signal
ATP, AMP, and Pi signal the energy status of the cell.
PFK-1 activity increases when the energy charge of the cell is low, that is, when ATP is needed, and decreases when the energy charge is high, that is, when ATP levels are elevated.[69]
How this happens
When ATP is produced faster than it is consumed, its intracellular concentration becomes elevated. Under these conditions, ATP binds to its allosteric site and inhibits PFK-1 by reducing the enzyme’s affinity for fructose 6-phosphate.
From a kinetic perspective, increased ATP concentration alters the relationship between enzyme activity and substrate concentration, changing the hyperbolic velocity curve of fructose 6-phosphate into a sigmoidal one and thereby increasing the Km for the substrate.
However, under most physiological conditions, intracellular ATP concentration does not vary significantly. For example, during vigorous exercise, ATP concentration in muscle may decrease by about 10% compared to resting levels, whereas the glycolytic rate changes much more than would be expected from such a small reduction.
When ATP consumption exceeds its production, concentrations of ADP and AMP rise, particularly AMP, due to the reaction catalyzed by adenylate kinase (EC 2.7.4.3), which forms ATP and AMP from two ADP molecules.
ADP + ADP ⇄ ATP + AMP
The equilibrium constant of the reaction is:
Keq = [ATP][AMP]/[ADP]2 = 0.44
Under physiological conditions, the concentrations of ADP and AMP are approximately 10% and often less than 1% of the ATP concentration, respectively. Therefore, considering that the total adenylate pool remains constant over the short term, even a small decrease in ATP concentration leads, due to adenylate kinase activity, to a much larger relative increase in AMP concentration. In turn, AMP acts by reversing the inhibition caused by ATP.
Thus, the activity of phosphofructokinase-1 depends on the cellular energy status:
- when ATP is plentiful, enzyme activity decreases;
- when AMP levels increase and ATP levels fall, enzyme activity increases.[9]
Why ADP isn’t a positive effector of PFK-1
There are two reasons.
First, when the cell’s energy charge decreases, ADP is used to regenerate ATP via the reaction catalyzed by adenylate kinase.
Second, as previously mentioned, even a small decrease in ATP leads to proportionally larger changes in ADP levels, and, especially, in AMP levels.[70]
Role of hydrogen ions
Hydrogen ions also inhibit PFK-1. This inhibition helps regulate the rate of glycolysis, preventing excessive lactate accumulation and a consequent drop in cellular pH.[71]
Role of citrate
Citrate is an allosteric inhibitor of PFK-1 that acts by enhancing the inhibitory effect of ATP.
It is the product of the first step of the citric acid cycle, a metabolic pathway that provides building blocks for biosynthetic processes and directs electrons into the mitochondrial electron transport chain for ATP synthesis via oxidative phosphorylation.
High cytosolic citrate levels indicate that energy demands are met and an abundance of biosynthetic precursors is available (exported from the mitochondria); in other words, the citric acid cycle is saturated.
Under such conditions, glycolysis, which feeds the cycle under aerobic conditions, can slow down, sparing glucose.
Therefore, it should be noted that PFK-1 functionally couples glycolysis to the citric acid cycle.[65][72]
Regulation of PFK-1 and FBPase-1 in the liver
In the liver, the central control point of glycolysis and gluconeogenesis is the substrate cycle between F6P and F1,6BP, catalyzed by PFK-1 and fructose 1,6-bisphosphatase.
The liver plays a pivotal role in maintaining blood glucose levels within the normal range.
When blood glucose levels drop, glucagon stimulates hepatic glucose production via both glycogenolysis and gluconeogenesis, while simultaneously signaling the liver to stop consuming glucose for its own energy needs.
Conversely, when blood glucose levels are high, insulin promotes glucose utilization for energy and the synthesis of glycogen and triglycerides.
In this context, the regulation of glycolysis and gluconeogenesis is mediated by fructose 2,6-bisphosphate (F2,6BP), a molecule that enables the liver to play a major role in regulating blood glucose levels and whose concentration is controlled by insulin and glucagon.[56]
Role of fructose 2,6-bisphosphate
By binding to its allosteric site on PFK-1, fructose 2,6-bisphosphate increases the affinity of the enzyme for fructose 6-phosphate, its substrate, while counteracting the inhibitory effect citrate and ATP.
It is remarkable that under physiological concentrations of substrates and both positive and negative allosteric effectors, PFK-1 would be virtually inactive in the absence of fructose 2,6-bisphosphate.
On the other hand, the binding of fructose 2,6-bisphosphate to fructose 1,6-bisphosphatase inhibits the enzyme, even in the absence of AMP, another of its allosteric inhibitors.
As a result of these effects, fructose 2,6-bisphosphate increases the net flux of glucose through glycolysis.[66][67][73]
Role of xylulose 5-phosphate
Xylulose 5-phosphate also plays a role in controlling carbon flux through glycolysis and gluconeogenesis. It is a product of the pentose phosphate pathway, whose levels in hepatocytes rise after the ingestion of a carbohydrate-rich meal. By activating protein phosphatase 2A, this metabolite ultimately increases fructose 2,6-bisphosphate levels, thereby enhancing glycolytic flux and reducing gluconeogenic flux.[74]
Regulation of pyruvate kinase activity
Another key control point for carbon flux through glycolysis and gluconeogenesis is the substrate cycle between phosphoenolpyruvate and pyruvate, catalyzed by pyruvate kinase in glycolysis and by the combined action of pyruvate carboxylase and phosphoenolpyruvate carboxykinase (EC 4.1.1.32) in gluconeogenesis.[9]
Allosteric regulation
All pyruvate kinase isozymes are allosterically inhibited by high concentrations of ATP, long-chain fatty acids, and acetyl-CoA, all indicators that the cell is in a high-energy state. Alanine, which can be synthesized from pyruvate through a transamination reaction, is also an allosteric inhibitor; its accumulation signals that biosynthetic precursors are abundant.
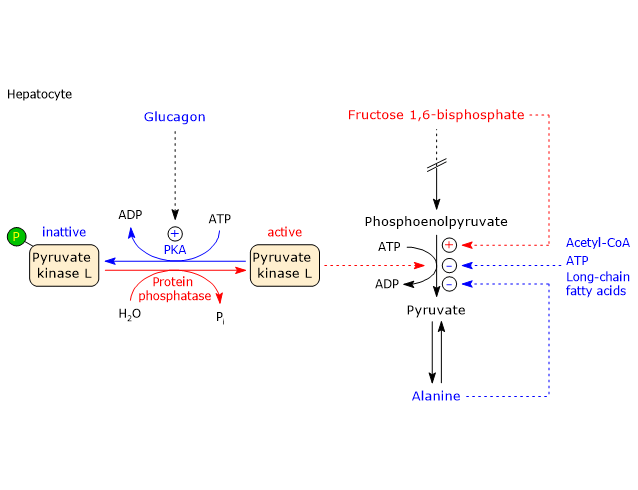
Conversely, pyruvate kinase is allosterically activated by fructose 1,6-bisphosphate, the product of the first committed step of glycolysis. F1,6BP thus ensures that pyruvate kinase activity keeps pace with the upstream flux of intermediates. It should be emphasized that, at physiological concentrations of PEP, ATP, and alanine, the enzyme would be almost completely inhibited in the absence of F1,6BP activation.[27][75]
Covalent regulation in the liver
The hepatic isoenzyme, but not the muscle isoenzyme, is also regulated by phosphorylation through:
- protein kinase A (PKA), activated by glucagon (or epinephrine via β-adrenergic receptors);
- calcium/calmodulin dependent protein kinase (CAMK), activated by epinephrine via α1-adrenergic receptors.
Phosphorylation of the enzyme decreases its activity by increasing the Km for PEP, thereby slowing glycolysis.
For example, when blood glucose levels are low, glucagon-induced phosphorylation reduces pyruvate kinase activity. The phosphorylated enzyme is also less responsive to activation by fructose 1,6-bisphosphate and more sensitive to inhibition by alanine and ATP.
Conversely, the dephosphorylated form of pyruvate kinase is more responsive to F1,6BP, that is, it requires a lower concentration for activation, and less sensitive to ATP and alanine. This mechanism allows the liver, under low-glucose conditions, to limit its own glucose utilization, preserving glucose for other organs such as the brain.
It should be noted, however, that the powerful activation by F1,6BP overrides the inhibitory effects of phosphorylation by PKA.
An increase in the insulin/glucagon ratio promotes dephosphorylation and enzyme activation.[9]
References
- ^ a b c d e f g h i j k l Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 8th Edition. W.H. Freeman and Company, 2021.
- ^ Alberts B., Johnson A., Lewis J., Morgan D., Raff M., Roberts K., Walter P. Molecular Biology of the Cell. 7th Edition. Garland Science, Taylor & Francis Group, 2022.
- ^ a b c d e f g h Heilman D., Woski S., Voet D., Voet J.G., Pratt C.W. Fundamentals of biochemistry: life at the molecular level. 6th Edition. Wiley, 2023.
- ^ Fruton J.S. A History of Biochemistry. Harvard University Press, 1982.
- ^ a b c d e f g h i j k Garrett R.H., Grisham C.M. Biochemistry. 7th Edition. Cengage Learning, 2023.
- ^ Buchner E., Rapp R. Alkoholische Gärung ohne Hefezellen. Berichte der Deutschen Chemischen Gesellschaft 1899;32(1):127-137. doi:10.1002/cber.18990320124
- ^ Kohler R. The background to Eduard Buchner’s discovery of cell-free fermentation. J Hist Biol 1971;4:35-61. doi:10.1007/BF00356976
- ^ Eduard Buchner – Biographical. NobelPrize.org. Nobel Prize Outreach 2025. Mon. 13 Oct 2025. https://www.nobelprize.org/prizes/chemistry/1907/buchner/biographical/
- ^ a b c d e f g h i j k Stipanuk M.H., Caudill M.A. Biochemical, physiological, and molecular aspects of human nutrition. 4th Edition. St. Louis: Elsevier, 2018.
- ^ Lu Z., Imlay J.A. When anaerobes encounter oxygen: mechanisms of oxygen toxicity, tolerance and defence. Nat Rev Microbiol 2021;19(12):774-785. doi:10.1038/s41579-021-00583-y
- ^ a b c d e f g h i j k l Berg J.M., Tymoczko J.L., Gregory J.G. Jr, Stryer L. Biochemistry. 9th Edition. W.H. Freeman and Company, 2019.
- ^ Plaxton W.C., Podestá F.E. The functional organization and control of plant respiration. Crit Rev Plant Sci 2006;25(2):159-198. doi:10.1080/07352680600563876
- ^ Hochachka P.W., Somero G.N. Biochemical adaptation: mechanism and process in physiological evolution. Oxford University Press, 2002. doi:10.1093/oso/9780195117028.001.0001
- ^ a b Naifeh N., Dimri M., Varacallo M.A. Biochemistry, Aerobic Glycolysis. [Updated 2023 Apr 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/sites/books/NBK470170/
- ^ Lunt S.Y., Vander Heiden M.G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 2011;27:441-64. doi:10.1146/annurev-cellbio-092910-154237
- ^ a b c d Wilson J.E. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol 2003;206(Pt 12):2049-57. doi:10.1242/jeb.00241
- ^ a b c d e f g h i j Atkins P.W., Ratcliffe R.G., Wormald M., De Paula J. Physical chemistry for the life sciences. 3rd Edition. Oxford University Press, 2023.
- ^ Mertens E. Pyrophosphate-dependent phosphofructokinase, an anaerobic glycolytic enzyme? FEBS Lett 1991;285(1):1-5. doi:10.1016/0014-5793(91)80711-b
- ^ Knowles J.R. Enzyme catalysis: not different, just better. Nature 1991;350(6314):121-4. doi:10.1038/350121a0
- ^ Sirover M.A. On the functional diversity of glyceraldehyde-3-phosphate dehydrogenase: biochemical mechanisms and regulatory control. Biochim Biophys Acta 2011;1810(8):741-51. doi:10.1016/j.bbagen.2011.05.010
- ^ Rojas-Pirela M., Andrade-Alviárez D., Rojas V., Kemmerling U., Cáceres A.J., Michels P.A., Concepción J.L., Quiñones W. Phosphoglycerate kinase: structural aspects and functions, with special emphasis on the enzyme from Kinetoplastea. Open Biol 2020;10(11):200302. doi:10.1098/rsob.200302
- ^ Levin S., Almo S.C., Satir B.H. Functional diversity of the phosphoglucomutase superfamily: structural implications. Protein Eng 1999;12(9):737-46. doi:10.1093/protein/12.9.737
- ^ Rapoport S., Luebering J. The formation of 2,3-diphosphoglycerate in rabbit erythrocytes: the existence of a diphosphoglycerate mutase. J Biol Chem 1950;183(2):507-516.
- ^ Webb K.L., Dominelli P.B., Baker S.E., Klassen S.A., Joyner M.J., Senefeld J.W., Wiggins C.C. Influence of high hemoglobin-oxygen affinity on humans during hypoxia. Front Physiol 2022;12:763933. doi:10.3389/fphys.2021.763933
- ^ Li M., Wu C., Yang Y., Zheng M., Yu S., Wang J., Chen L., Li H. 3-Phosphoglycerate dehydrogenase: a potential target for cancer treatment. Cell Oncol (Dordr) 2021;44(3):541-556. doi:10.1007/s13402-021-00599-9
- ^ Lebioda L., Stec B. Mechanism of enolase: the crystal structure of enolase-Mg2+-2-phosphoglycerate/phosphoenolpyruvate complex at 2.2-A resolution. Biochemistry 1991;30(11):2817-22. doi:10.1021/bi00225a012
- ^ a b Schormann N., Hayden K.L., Lee P., Banerjee S., Chattopadhyay D. An overview of structure, function, and regulation of pyruvate kinases. Protein Sci 2019;28(10):1771-1784. doi:10.1002/pro.3691
- ^ Yang Y., Sauve A.A. NAD+ metabolism: bioenergetics, signaling and manipulation for therapy. Biochim Biophys Acta 2016;1864(12):1787-1800. doi:10.1016/j.bbapap.2016.06.014
- ^ Gray L.R., Tompkins S.C., Taylor E.B. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci 2014;71(14):2577-604. doi:10.1007/s00018-013-1539-2
- ^ Rogatzki M.J., Ferguson B.S., Goodwin M.L., Gladden L.B. Lactate is always the end product of glycolysis. Front Neurosci 2015;9:22. doi:10.3389/fnins.2015.00022
- ^ Geison G.L. The Private Science of Louis Pasteur. Princeton University Press, 1995.
- ^ a b c d e Rodwell V.W., Bender D.A., Botham K.M., Kennelly P.J., Weil P.A. Harper’s Illustrated Biochemistry. 31st Edition. McGraw-Hill, 2018.
- ^ Dong S., Qian L., Cheng Z., Chen C., Wang K., Hu S., Zhang X., Wu T. Lactate and myocardiac energy metabolism. Front Physiol 2021;12:715081. doi:10.3389/fphys.2021.715081
- ^ National Research Council (US) Panel on the Applications of Biotechnology to Traditional Fermented Foods. Applications of Biotechnology to Fermented Foods: Report of an Ad Hoc Panel of the Board on Science and Technology for International Development. Washington (DC): National Academies Press (US); 1992. 5, Lactic Acid Fermentations. Available from: https://www.ncbi.nlm.nih.gov/books/NBK234703/
- ^ Dzialo M.C., Park R., Steensels J., Lievens B., Verstrepen K.J. Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiol Rev 2017;41(Supp_1):S95-S128. doi:10.1093/femsre/fux031
- ^ Maicas S. The role of yeasts in fermentation processes. Microorganisms 2020;8(8):1142. doi:10.3390/microorganisms8081142
- ^ Patel M.S., Nemeria N.S., Furey W., and Jordan F. The pyruvate dehydrogenase complexes: structure-based function and regulation. J Biol Chem 2014;289(24):16615-16623. doi:10.1074/jbc.R114.563148
- ^ Liu H., Wang S., Wang J., Guo X., Song Y., Fu K., Gao Z., Liu D., He W., Yang L.L. Energy metabolism in health and diseases. Signal Transduct Target Ther 2025;10(1):69. doi:10.1038/s41392-025-02141-x
- ^ Borst P. The malate-aspartate shuttle (Borst cycle): How it started and developed into a major metabolic pathway. IUBMB Life 2020;72(11):2241-2259. doi:10.1002/iub.2367
- ^ a b Ahmad M., Wolberg A., Kahwaji C.I. Biochemistry, Electron Transport Chain. [Updated 2023 Sep 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526105/
- ^ Chaudhry R., Varacallo M.A. Biochemistry, Glycolysis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482303/
- ^ DeBerardinis R.J., Chandel N.S. Fundamentals of cancer metabolism. Sci Adv 2016;2(5):e1600200. doi:10.1126/sciadv.1600200
- ^ Melkonian E.A., Schury M.P. Biochemistry, Anaerobic Glycolysis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK546695/
- ^ Chandel N.S. Glycolysis. Cold Spring Harb Perspect Biol. 2021;13(5):a040535. doi:10.1101/cshperspect.a040535
- ^ Chandel N.S. Carbohydrate Metabolism. Cold Spring Harb Perspect Biol 2021;13(1):a040568. doi:10.1101/cshperspect.a040568
- ^ Kiela P.R., Ghishan F.K. Physiology of intestinal absorption and secretion. Best Pract Res Clin Gastroenterol 2016;30(2):145-59. doi:10.1016/j.bpg.2016.02.007
- ^ a b Dholariya S.J., Orrick J.A. Biochemistry, Fructose Metabolism. [Updated 2022 Oct 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK576428/
- ^ a b Hannou S.A., Haslam D.E., McKeown N.M., Herman M.A. Fructose metabolism and metabolic disease. J Clin Invest 2018;128(2):545-555. doi:10.1172/JCI96702
- ^ Legeza B., Marcolongo P., Gamberucci A., Varga V., Bánhegyi G., Benedetti A., Odermatt A. Fructose, glucocorticoids and adipose tissue: implications for the metabolic syndrome. Nutrients 2017;9(5):426. doi:10.3390/nu9050426
- ^ Frey P.A. The Leloir pathway: a mechanistic imperative for three enzymes to change the stereochemical configuration of a single carbon in galactose. FASEB J 1996;10(4):461-70. doi:10.1096/fasebj.10.4.8647345
- ^ Conte F., van Buuringen N., Voermans N.C., Lefeber D.J. Galactose in human metabolism, glycosylation and congenital metabolic diseases: time for a closer look. Biochim Biophys Acta Gen Subj 2021;1865(8):129898. doi:10.1016/j.bbagen.2021.129898
- ^ Succoio M., Sacchettini R., Rossi A., Parenti G., Ruoppolo M. Galactosemia: biochemistry, molecular genetics, newborn screening, and treatment. Biomolecules 2022;12(7):968. doi:10.3390/biom12070968
- ^ Sharma V., Ichikawa M., Freeze H.H. Mannose metabolism: more than meets the eye. Biochem Biophys Res Commun 2014;453(2):220-8. doi:10.1016/j.bbrc.2014.06.021
- ^ a b de la Iglesia N., Mukhtar M., Seoane J., Guinovart J.J., Agius L. The role of the regulatory protein of glucokinase in the glucose sensory mechanism of the hepatocyte. J Biol Chem 2000;275(14):10597-10603. doi: 10.1074/jbc.275.14.10597
- ^ Zhang Y., Lin Z., Wang M., Lin H. Selective usage of isozymes for stress response. ACS Chem Biol 2018;13(11):3059-3064. doi:10.1021/acschembio.8b00767
- ^ a b Han H.S., Kang G., Kim J.S., Choi B.H., Koo S.H. Regulation of glucose metabolism from a liver-centric perspective. Exp Mol Med 2016;48(3):e218. doi:10.1038/emm.2015.122
- ^ Huang S., Czech M.P. The GLUT4 glucose transporter. Cell Metab 2007;5(4):237-52. doi:10.1016/j.cmet.2007.03.006
- ^ Matschinsky F.M., Magnuson M.A., Zelent D., Jetton T.L., Doliba N., Han Y., Taub R., Grimsby J. The network of glucokinase-expressing cells in glucose homeostasis and the potential of glucokinase activators for diabetes therapy. Diabetes 2006;55(1):1-12. doi:diabetes.55.01.06.db05-0926
- ^ Sun B., Chen H., Xue J., Li P., Fu X. The role of GLUT2 in glucose metabolism in multiple organs and tissues. Mol Biol Rep 2023;50(8):6963-6974. doi:10.1007/s11033-023-08535-w
- ^ a b Adeva-Andany M.M., Pérez-Felpete N., Fernández-Fernández C., Donapetry-García C., Pazos-García C. Liver glucose metabolism in humans. Biosci Rep 2016;36(6):e00416. doi:10.1042/BSR20160385
- ^ Abu Aqel Y., Alnesf A., Aigha I.I., Islam Z., Kolatkar P.R., Teo A., Abdelalim E.M. Glucokinase (GCK) in diabetes: from molecular mechanisms to disease pathogenesis. Cell Mol Biol Lett. 2024 Sep 8;29(1):120. doi:10.1186/s11658-024-00640-3
- ^ Kaminski M.T., Schultz J., Waterstradt R., Tiedge M., Lenzen S., Baltrusch S. Glucose-induced dissociation of glucokinase from its regulatory protein in the nucleus of hepatocytes prior to nuclear export. Biochim Biophys Acta 2014;1843(3):554-564. doi:10.1016/j.bbamcr.2013.12.002
- ^ Sternisha S.M., Miller B.G. Molecular and cellular regulation of human glucokinase. Arch Biochem Biophys 2019;663:199-213. doi:10.1016/j.abb.2019.01.011
- ^ Matschinsky F.M., Wilson D.F. The central role of glucokinase in glucose homeostasis: a perspective 50 years after demonstrating the presence of the enzyme in islets of Langerhans. Front Physiol 2019;10:148. doi:10.3389/fphys.2019.00148
- ^ a b Mor I., Cheung E.C., Vousden K.H. Control of glycolysis through regulation of PFK1: old friends and recent additions. Cold Spring Harb Symp Quant Biol 2011;76:211-6. doi:10.1101/sqb.2011.76.010868
- ^ a b Van Schaftingen E., and Hers H-G. Inhibition of fructose-1,6-bisphosphatase by fructose-2,6-bisphosphate. Proc Natl Acad Sci USA 1981;78(5):2861-2863. doi:10.1073/pnas.78.5.2861
- ^ a b Van Schaftingen E., Jett M-F., Hue L., and Hers H-G. Control of liver 6-phosphofructokinase by fructose 2,6-bisphosphate and other effectors. Proc Natl Acad Sci USA 1981;78(6):3483-3486. doi:10.1073/pnas.78.6.3483
- ^ Lynch E.M., Hansen H., Salay L., Cooper M., Timr S., Kollman J.M., Webb B.A. Structural basis for allosteric regulation of human phosphofructokinase-1. Nat Commun 2024;15(1):7323. doi:10.1038/s41467-024-51808-6
- ^ Jenkins C.M., Yang J., Sims H.F., Gross R.W. Reversible high affinity inhibition of phosphofructokinase-1 by acyl-CoA: a mechanism integrating glycolytic flux with lipid metabolism. J Biol Chem 2011;286(14):11937-50. doi:10.1074/jbc.M110.203661
- ^ Atkinson D.E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry 1968;7(11):4030-4. doi:10.1021/bi00851a033
- ^ Lešnik S., Konc J., Vodopivec T., Čamernik K., Karolina Potokar U., Legiša M. Small-molecule inhibitors of 6-phosphofructo-1-kinase simultaneously suppress lactate and superoxide generation in cancer cells. PLoS One 2025;20(5):e0321998. doi:10.1371/journal.pone.0321998
- ^ Usenik A., Legiša M. Evolution of allosteric citrate binding sites on 6-phosphofructo-1-kinase. PLoS One 2010;5(11):e15447. doi:10.1371/journal.pone.0015447
- ^ Chaekal O.K., Boaz J.C., Sugano T., Harris R.A. Role of fructose 2,6-bisphosphate in the regulation of glycolysis and gluconeogenesis in chicken liver. Arch Biochem Biophys 1983;225(2):771-8. doi:10.1016/0003-9861(83)90088-7
- ^ Kabashima T., Kawaguchi T., Wadzinski B.E., Uyeda K. Xylulose 5-phosphate mediates glucose-induced lipogenesis by xylulose 5-phosphate-activated protein phosphatase in rat liver. Proc Natl Acad Sci USA 2003;100:5107-5112. doi:10.1073/pnas.0730817100
- ^ Jurica M.S., Mesecar A., Heath P.J., Shi W., Nowak T., Stoddard B.L. The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate. Structure 1998;6(2):195-210. doi:10.1016/s0969-2126(98)00021-5