Olive oil is a liquid fat obtained by pressing whole olives, the fruit of the olive tree (Olea europaea).[1][2]
It is a staple ingredient in Mediterranean diet and is widely used for cooking, salad dressings, and other culinary purposes. Olive oil is also known for its health benefits, as it is rich in monounsaturated fats and antioxidants.[3]
From a chemical point of view, two fractions can be identified in olive oil, depending on their behavior in the presence of heat and strong alkaline solutions:
- the saponifiable fraction, which represents 98−99% of the total weight, is composed of lipids that form soaps under the above conditions;
- the unsaponifiable fraction, which represents the remaining 1−2% of the total weight, composed of substances that do not form soaps under the above conditions.[4][5]
Contents
Saponifiable fraction
It is composed of saturated and unsaturated fatty acids, esterified almost entirely to glycerol to form triglycerides or triacylglycerols. To a much lesser extent, diglycerides (or diacylglycerols), monoglycerides (or monoacylglycerols), and free fatty acids are also present.[4]
Unsaturated fatty acids make up 75 to 85% of the total, while saturated fatty acids account for 15–25%.
Oleic acid (O) and linoleic acid (L) are the most abundant; palmitoleic acid, heptadecenoic acid, gadoleic acid, and alpha-linolenic acid (Ln) are present in lower or trace amounts.[6]
| Fatty acid | Number of carbons | Allowable range (%) |
|---|---|---|
| Myristic acid | C14:0 | <0.03 |
| Palmitic acid | C16:0 | 7.5−20 |
| Palmitoleic acid | C16:1 | 0.3−3.5 |
| Heptadecanoic acid | C17:0 | ≤0.3 |
| Heptadecenoic acid | C17:1 | ≤0.3 |
| Stearic acid | C18:0 | 0.5−5.0 |
| Oleic acid | C18:1 | 55.0−83.0 |
| Linoleic acid | C18:2 | 2.5−21.0 |
| α-Linolenic acid | C18:3 | ≤1.0 |
| Arachidic acid | C20:0 | ≤0.6 |
| Gadoleic acid | C20:1 | ≤0.4 |
| Behenic acid | C22:0 | ≤0.2 |
| Lignoceric acid | C24:0 | ≤0.2 |
Unsaturated and saturated fatty acids
Oleic acid is the major fatty acid in olive oil.[7] According to the rules laid down by the International Olive Oil Council (IOOC), its concentration must range from 55 to 83% of total fatty acids.
Linoleic acid is the most abundant polyunsaturated fatty acid in olive oil; its concentration must vary between 2.5 and 21% (IOOC). Because of its high degree of unsaturation, it is prone to oxidation; this means that an oil rich in linoleic acid becomes rancid more easily and therefore has a shorter shelf life.[8]
In Mediterranean cuisine, olive oil is the main source of fat; therefore, oleic acid (among monounsaturated fatty acids) and linoleic acid (among polyunsaturated fatty acids) are the most abundant fatty acids.
α-Linolenic acid must be present in very small amounts, according to IOOC standards (≤1 percent). It is an omega-3 polyunsaturated fatty acid that may have health benefits. However, because of its high degree of unsaturation, higher than that of linoleic acid, it is very susceptible to oxidation and therefore promotes rancidity in olive oil containing it.
Saturated fatty acids make up 15 to 25% of the total fatty acids.
Palmitic acid (P, 5−20%), and stearic acid (S, 0.5−5%), are the most abundant saturated fatty acids; myristic, heptadecanoic, arachidic, behenic, and lignoceric acid may be present in trace amounts.
The presence of fatty acids that should be absent or are found in abnormal amounts is a marker of adulteration with other vegetable oils. In this regard, particular attention is paid to myristic, arachidic, behenic, lignoceric, gadoleic, and α-linolenic acids, whose limits are set by the IOOC.[6]
Factors influencing fatty acid profile
Fatty acid composition is influenced by several factors:
- the climate;
- the latitude;
- the zone of production.
Italian, Spanish, and Greek olive oils are high in oleic acid and low in palmitic and linoleic acids, while Tunisian olive oils are high in palmitic and linoleic acids but lower in oleic acid. Therefore, oils can be divided into two groups:
Other influencing factors include:
It should be noted that oleic acid is formed first in the fruit, and data seem to indicate a competitive relationship between oleic acid and palmitic, palmitoleic, and linoleic acids.[8]
Triglycerides
As previously mentioned, fatty acids in olive oil are almost entirely present as triglycerides.
In small percentages, they are also present as diglycerides, monoglycerides, and in free form.[12]
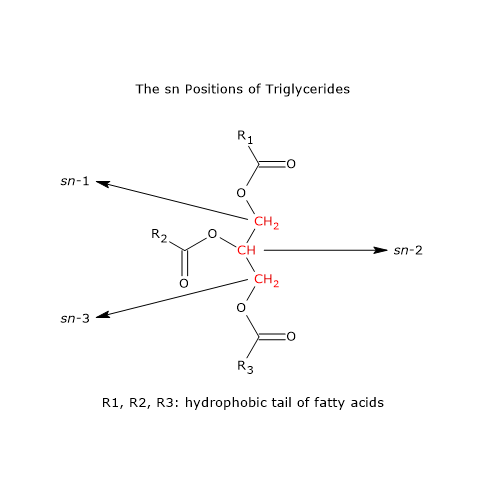
During triglyceride biosynthesis, thanks to the action of specific enzymes, only about 2% of glycerol molecules bind palmitic acid at the sn-2 position. The percentage of stearic acid in the sn-2 position is also very low. For the most part, the sn-2 position is occupied by oleic acid.[4][13]
Conversely, in oils that have undergone nonenzymatic esterification, the percentage of palmitic acid in the sn-2 position increases significantly.[11]
Note: sn = stereospecific numbering
The relative abundance of olive oil triglycerides is well characterized and standardized by the International Olive Oil Council. Among the triglycerides present in significant proportions in olive oil, the following are the most abundant:
- OOO: 40−59%;
- POO: 12−20%;
- OOL: 12.5−20%;
- POL: 5.5−7%;
- SOO: 3−7%.
POP, POS, OLnL, OLnO, PLL, and PLnO are present in smaller amounts.[6]
Trilinolein (LLL) is a triglyceride that contains three molecules of linoleic acid; its low content is an indicator of good-quality oil.
Triglycerides containing three saturated fatty acids or three molecules of α-linolenic acid have not been reported.[14]
Diglycerides and monoglycerides
Their presence is due to incomplete synthesis and/or partial hydrolysis of triglycerides.
The content of diglycerides in virgin olive oil ranges from 1 to 2.8%. 1,2-Diglycerides prevail in fresh olive oil, representing over 80% of total diglycerides.[15]
During storage, isomerization occurs with a progressive increase in the more stable 1,3-isomers, which after about 10 months become the predominant form. Therefore, the 1,2/1,3-diglyceride ratio may be used as an indicator of the age of the oil.
Monoglycerides are present in amounts lower than diglycerides (<0.25%), with 1-monoglycerides being far more abundant than 2-monoglycerides.[16]
Unsaponifiable fractions
This fraction is composed of a large number of different molecules, which play a key nutritional role, as they contribute significantly to the health benefits of olive oil. Furthermore, they are responsible for the stability and taste of olive oil and are also used to detect adulteration with other vegetable oils.[4]
It includes tocopherols, sterols, polyphenols, pigments, hydrocarbons, aromatic and aliphatic alcohols, triterpene acids, waxes, and other minor constituents.[17]
Their content is influenced by factors similar to those affecting fatty acid composition, such as:
- the cultivar;
- the degree of ripeness of the olives;
- the production area;
- the crop year and harvesting practices;
- the storage time of the olives;
- the oil extraction process;
- the storage conditions of the oil.[11]
It should be noted that many of these compounds are not present in refined olive oils, as they are removed during the refining process.[11]
Polyphenols
Polyphenols make up 18 to 37% of the unsaponifiable fraction.
They are a very heterogeneous group of molecules with nutritional and organoleptic properties; for example, oleuropein and hydroxytyrosol give olive oil its bitter and pungent taste.[5][18]
For a more detailed discussion, see the article Polyphenols in olive oil.
Hydrocarbons
Hydrocarbons make up 30 to 50% of the unsaponifiable fraction.
Squalene and β-carotene are the main compounds.[19]
Squalene, first isolated from shark liver, is the major constituent of the unsaponifiable fraction, accounting for more than 90% of the hydrocarbons. Its concentration ranges from 200 to 7,500 mg/kg of olive oil.[20]
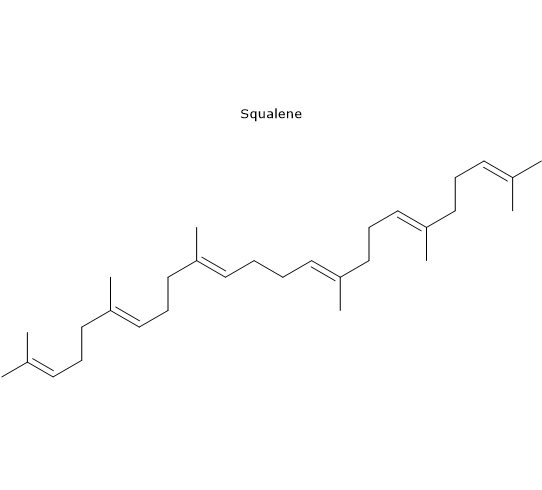
It is an intermediate in the biosynthesis of the four-ring structure of steroids and appears to be responsible for several of the health benefits attributed to olive oil.[21]
In the hydrocarbon fraction of virgin olive oil, n-paraffins, diterpene and triterpene hydrocarbons, and isoprenoid polyolefins are also found.[22]
Sterols
Sterols are important lipids of olive oil. They are:
- linked to many health benefits for consumers;
- important for the quality of the oil;
- widely used to verify its authenticity.
In this regard, it should be emphasized that sterols are species-specific molecules. For example, the presence of high concentrations of brassicasterol, a sterol typically found in the Brassicaceae (Cruciferae) family, such as rapeseed, indicates adulteration of olive oil with canola oil.[11]
Four classes of sterols are present in olive oil: common sterols, 4-methylsterols, triterpene alcohols, and triterpene dialcohols.[22]
Their total content ranges from 1,000 mg/kg (the minimum value required by IOOC standards) to 2,000 mg/kg. The lowest values are found in refined oils, since the refining process may cause losses of up to 25%.[6][23][24]
Common sterols or 4-α-desmethylsterols
Common sterols are present mainly in free and esterified forms; however, they have also been detected as lipoproteins and steryl glucosides.
The main molecules are β-sitosterol, which makes up 75–90% of the total sterols; Δ5-avenasterol, 5–20%, and campesterol, about 4%.
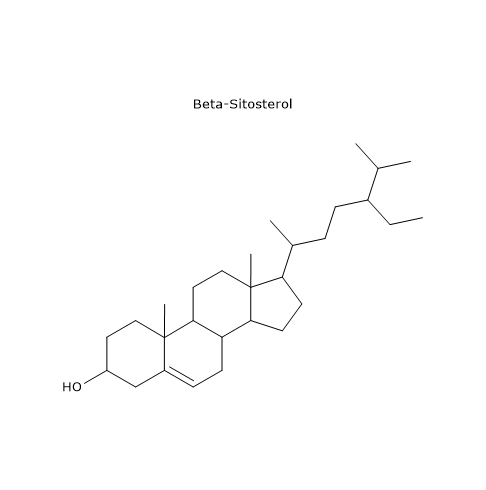
Other components found in smaller or trace amounts include stigmasterol (≈ 2%), cholesterol, brassicasterol, and ergosterol.[24]
4-Methylsterols
These compounds are intermediates in sterol biosynthesis and are present in both free and esterified forms.[24]
They occur in small amounts, much lower than those of common sterols and triterpene alcohols, ranging from 50 to 360 mg/kg.
The main molecules are obtusifoliol, cycloeucalenol, citrostadienol, and gramisterol.[22]
Triterpene alcohols or 4,4-dimethylsterols
This is a complex class of sterols, present in both free and esterified forms.
They are found in amounts ranging from 350 to 1,500 mg/kg.
The main components are β-amyrin, 24-methylenecycloartanol, cycloartenol, and butyrospermol.
Other molecules present in lower or trace amounts include cyclosadol, cyclobranol, germanicol, and dammaradienol.[22]
Triterpene dialcohols
The main triterpene dialcohols found in olive oil are erythrodiol and uvaol.
Erythrodiol occurs in both free and esterified forms; in virgin olive oil, its content ranges from 19 to 69 mg/kg, and the free form is generally lower than 50 mg/kg.[22][23]
Tocopherols
Tocopherols make up 2 to 3% of the unsaponifiable fraction and include vitamin E.[4]
Of the eight E-vitamers, α-tocopherol represents about 90% of total tocopherols in virgin olive oil. It is present in the free form and in variable amounts, generally exceeding 100 mg/kg of olive oil.[25]
Thanks to its in vivo antioxidant properties, its presence is a protective factor for health.[26]
The α-tocopherol concentration appears to be related to the high levels of chlorophylls and the concurrent need for singlet oxygen deactivation.[27]
β-Tocopherol, δ-tocopherol, and γ-tocopherol are usually present in small amounts.[22]
Pigments
In this group we find chlorophylls and carotenoids.
In olive oil, chlorophylls are present as pheophytins, mainly pheophytin A, a chlorophyll molecule in which magnesium has been replaced by two hydrogen ions, and they confer the characteristic green color to olive oil.
They act as photosensitizers, contributing to the photooxidation of the oil itself.[28][29]
β-Carotene and lutein are the main carotenoids in olive oil. Several xanthophylls are also present, such as antheraxanthin, β-cryptoxanthin, luteoxanthin, mutatoxanthin, neoxanthin, and violaxanthin.[30]
The color of olive oil results from the presence of chlorophylls and carotenoids and from their combined green and yellow hues.[31]
Triterpene acids
These are important components of olives and are present in trace amounts in the oil.
Oleanolic and maslinic acids are the main triterpene acids in virgin olive oil; they are found in the olive husk and are extracted in small quantities during processing.[32]
Aliphatic and aromatic alcohols
The most important are fatty alcohols and diterpene alcohols.
Aliphatic alcohols contain between 20 and 30 carbon atoms and are found mainly inside the olive stones, from which they are partially extracted during milling.[4]
Fatty alcohols
These are linear saturated alcohols containing more than 16 carbon atoms.
They occur in both free and esterified forms and, in virgin olive oil, are generally present at concentrations not exceeding 250 mg/kg.
Docosanol, tetracosanol, hexacosanol, and octacosanol are the main fatty alcohols in olive oil, with tetracosanol and hexacosanol being the most abundant.
Waxes, which are minor constituents of olive oil, are esters of fatty alcohols with fatty acids, mainly palmitic and oleic acids.
They can be used as a criterion to distinguish between different types of oils; for example, according to IOOC standards, they must be present in virgin and extra virgin olive oil at levels below 150 mg/kg.[11]
Diterpene alcohols
Geranylgeraniol and phytol are two acyclic diterpene alcohols, present in both free and esterified forms.
Among the esters present in the wax fraction of extra virgin olive oil, oleate, eicosenoate, eicosanoate, docosanoate, and tetracosanoate have been identified, mainly as phytyl derivatives.[11]
Volatile compounds
More than 280 volatile compounds have been identified in olive oil, including hydrocarbons (the most abundant fraction), alcohols, aldehydes, ketones, esters, acids, ethers, and many others.
However, only about 70 compounds are present at levels above the perception threshold, beyond which they contribute to the aroma of virgin olive oil.[33][34]
Minor components
Among the minor components of olive oil are phospholipids, mainly phosphatidylserine, phosphatidylethanolamine, phosphatidylcholine, and phosphatidylinositol.
In unfiltered oils, trace amounts of proteins may also be found.[4]
References
- ^ National Center for Biotechnology Information. PubChem Compound Summary for Olive Oil. https://pubchem.ncbi.nlm.nih.gov/compound/Olive-Oil. Accessed Nov. 13, 2025.
- ^ Codex Alimentarius Commission. Codex standard for olive oils and olive pomace oils (CXS 33-1981). Rome: FAO/WHO; 2019. Available from: https://www.fao.org/fao-who-codexalimentarius/
- ^ Mazzocchi A., Leone L., Agostoni C., Pali-Schöll I. The secrets of the Mediterranean diet. Does [only] olive oil matter? Nutrients 2019;11(12):2941. doi:10.3390/nu11122941
- ^ a b c d e f g Gunstone F.D. Vegetable oils in food technology: composition, properties and uses. 2nd Edition. Wiley J. & Sons, Inc., Publication, 2011.
- ^ a b Bulotta S., Celano M., Lepore S.M., Montalcini T., Pujia A., Russo D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: focus on protection against cardiovascular and metabolic diseases. J Transl Med 2014;12:219. doi:10.1186/s12967-014-0219-9
- ^ a b c d International Olive Council. Trade standard applying to olive oils and olive-pomace oils (COI/T.15/NC No 3/Rev. 16, 2021). Madrid: International Olive Council; June 2021. Available from: https://www.internationaloliveoil.org/wp-content/uploads/2021/07/COI-T15-NC3-REV-16-2021-_ENG.pdf
- ^ a b Tomé-Rodríguez S., Barba-Palomeque F., Ledesma-Escobar C.A., Miho H., Díez C.M., Priego-Capote F. Influence of genetic and interannual factors on the fatty acids profile of virgin olive oil. Food Chem 2023;422:136175. doi:10.1016/j.foodchem.2023.136175
- ^ a b Hernández M.L., Sicardo M.D., Belaj A., Martínez-Rivas J.M. The oleic/linoleic acid ratio in olive (Olea europaea L.) fruit mesocarp is mainly controlled by OeFAD2-2 and OeFAD2-5 genes together with the different specificity of extraplastidial acyltransferase enzymes. Front Plant Sci 2021;12:653997. doi:10.3389/fpls.2021.653997
- ^ Cetinkaya H., Kulak M. Soil characteristics influence the fatty acid profile of olive oils. Scientific Papers. Series B, Horticulture. 2016;60:53-57. Available at: https://www.researchgate.net/publication/387363822
- ^ Ben Hmida R., Gargouri B., Chtourou F., Sevim D., Bouaziz M. Fatty acid and triacyglycerid as markers of virgin olive oil from mediterranean region: traceability and chemometric authentication. Eur Food Res Technol 2022;248:1-16. doi:10.1007/s00217-022-04002-1
- ^ a b c d e f g Boskou D. Olive oil: chemistry and technology. 3rd Edition, AOCS Press, 2015.
- ^ Lukić I., Da Ros A., Guella G., Camin F., Masuero D., Mulinacci N., Vrhovsek U., Mattivi F. Lipid profiling and stable isotopic data analysis for differentiation of extra virgin olive oils based on their origin. Molecules 2019;25(1):4. doi:10.3390/molecules25010004
- ^ Karupaiah T., Sundram K. Effects of stereospecific positioning of fatty acids in triacylglycerol structures in native and randomized fats: a review of their nutritional implications. Nutr Metab (Lond) 2007;4:16. doi:10.1186/1743-7075-4-16
- ^ Jawad M.A., Jawhar A., Dakah A., Ejbara M.G. Effect of maturity stages on triglyceride and fatty acid profiles in olive oil from Zaity and Kaissy varieties grown in Syria. Sci Rep 2025;15(1):35935. doi:10.1038/s41598-025-20162-y
- ^ International Olive Council. Determination of composition of triacylglycerols and content of di-acylglycerols by capillary gas chromatography. COI/T.20/Doc. No 32. Madrid: International Olive Council; November 2013. Available from https://www.internationaloliveoil.org/wp-content/uploads/2022/10/COI-T20-Doc.32-2013-Eng.pdf
- ^ Caponio F., Bilancia M.T., Pasqualone A., Sikorska E., Gomes T. Influence of the exposure to light on extra virgin olive oil quality during storage. Eur Food Res Technol 2005;221:92-98. doi:10.1007/s00217-004-1126-8
- ^ Talarico I.R., Bartella L., Rocio-Bautista P., Di Donna L., Molina-Diaz A., Garcia-Reyes J.F. Paper spray mass spectrometry profiling of olive oil unsaponifiable fraction for commercial categories classification. Talanta 2024;267:125152. doi:10.1016/j.talanta.2023.125152
- ^ Servili M., Sordini B., Esposto S., Urbani S., Veneziani G., Di Maio I., Selvaggini R. and Taticchi A. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants 2014;3:1-23. doi:10.3390/antiox3010001
- ^ Barp L., Miklavčič Višnjevec A., Moret S. Analytical determination of Squalene in extra virgin olive oil and olive processing by-products, and its valorization as an ingredient in functional food – A critical review. Molecules 2024;29(21):5201. doi:10.3390/molecules29215201
- ^ Hernández M.L., Muñoz-Ocaña C., Posada P., et al. Functional characterization of four olive squalene synthases with respect to the squalene content of the virgin olive oil. J Agric Food Chem 2023;71(42):15701-15712. doi:10.1021/acs.jafc.3c05322
- ^ Ibrahim N., Fairus S., Zulfarina M.S., Naina Mohamed I. The efficacy of squalene in cardiovascular disease risk – A systematic review. Nutrients 2020;12(2):414. doi:10.3390/nu12020414
- ^ a b c d e f Jimenez-Lopez C., Carpena M., Lourenço-Lopes C., Gallardo-Gomez M., Lorenzo J.M., Barba F.J., Prieto M.A., Simal-Gandara J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020;9(8):1014. doi:10.3390/foods9081014
- ^ a b International Olive Council. Determination of the composition and content of sterols and triterpene dialcohols by capillary gas chromatography. COI/T.20/Doc. No 30/Rev. 2 – 2017. Madrid: International Olive Council; 2017. Available from: https://www.internationaloliveoil.org/wp-content/uploads/2022/10/COI-T20-Doc.30-rev.-2-2017-Eng.pdf
- ^ a b c Lukić M., Lukić I., Krapac M., Sladonja B., Piližota V. Sterols and triterpene diols in olive oil as indicators of variety and degree of ripening. Food Chem 2013;136(1):251-8. doi:10.1016/j.foodchem.2012.08.005
- ^ Beltrán G., Jiménez A., del Rio C., Sánchez S., Martínez L. Variability of vitamin E in virgin olive oil by agronomical and genetic factors. J Food Compos Anal 2010;23(6):633-639. doi:10.1016/j.jfca.2010.03.003
- ^ Sánchez-Villegas A., Sánchez-Tainta A. Virgin olive oil. In the prevention of cardiovascular disease through the
Mediterranean diet. Academic Press. Cambridge, MA, USA, 2018;59-87. - ^ Grams G.W., Eskins K. Dye-sensitized photooxidation of tocopherols. Correlation between singlet oxygen reactivity and vitamin E activity. Biochemistry 1972;11(4):606-8. doi:10.1021/bi00754a020
- ^ Giuliani A., Cerretani L., Cichelli A. Chlorophylls in olive and in olive oil: chemistry and occurrences. Crit Rev Food Sci Nutr 2011;51(7):678-90. doi:10.1080/10408391003768199
- ^ Díez-Betriu A., Bustamante J., Romero A., Ninot A., Tres A., Vichi S., Guardiola F. Effect of the storage conditions and freezing speed on the color and chlorophyll profile of premium extra virgin olive oils. Foods 2023;12(1):222. doi:10.3390/foods12010222
- ^ Gandul-Rojas B., Roca M., Gallardo-Guerrero L. Chlorophylls and carotenoids in food products from olive tree. In: Boskou D., Clodoveo M.L. Products from Olive Tree. IntechOpen, London, 2016. doi:10.5772/64688
- ^ Borello E., Roncucci D., Domenici V. Study of the evolution of pigments from freshly pressed to ‘on-the-shelf’ extra-virgin olive oils by means of near-UV visible spectroscopy. Foods 2021;10(8):1891. doi:10.3390/foods10081891
- ^ Sánchez-Quesada C., López-Biedma A., Warleta F., Campos M., Beltrán G., Gaforio J.J. Bioactive properties of the main triterpenes found in olives, virgin olive oil, and leaves of Olea europaea. J Agric Food Chem 2013;61(50):12173-82. doi:10.1021/jf403154e
- ^ Nardella M., Moscetti R., Bedini G., Bandiera A., Chakravartula S.S.N., Massantini R. Impact of traditional and innovative malaxation techniques and technologies on nutritional and sensory quality of virgin olive oil – A review. Food Chem Adv
Volume 2023;2:100163. doi:10.1016/j.focha.2022.100163 - ^ Angerosa F., Servili M., Selvaggini R., Taticchi A., Esposto S., Montedoro G. Volatile compounds in virgin olive oil: occurrence and their relationship with the quality. J Chromatogr A 2004;1054(1-2):17-31. doi:10.1016/j.chroma.2004.07.093