Carbohydrate loading is a good strategy to increase fuel stores in muscles before the start of the competition.
What does the muscle “eat” during endurance exercise?
Muscle “eats” carbohydrates, in the form of liver and muscle glycogen, carbohydrates ingested during the exercise or just before that, and fat.
During endurance exercise, the most likely contributors to fatigue are dehydration and carbohydrate depletion, especially of muscle and liver glycogen.
To prevent the “crisis” due to the depletion of muscle and liver carbohydrates, it is essential having high glycogen stores before the start of the activity.
What does affect glycogen stores?
- The diet in the days before the competition.
- The level of training (well-trained athletes synthesize more glycogen and have potentially higher stores, because they have more efficient enzymes).
- The activity in the day of the competition and the days before (if muscle doesn’t work it doesn’t lose glycogen). Therefore, it is better to do light trainings in the days before the competition, not to deplete glycogen stores, and to take care of nutrition.
The “Swedish origin” of carbohydrate loading
Very high muscle glycogen levels, the so-called glycogen supercompensation, can improve performance, i.e. time to complete a predetermined distance, by 2-3% in the events lasting more than 90 minutes, compared with low to normal glycogen, while benefits seem to be little or absent when the duration of the event is less than 90 min.
Well-trained athletes can achieve glycogen supercompensation without the depletion phase prior to carbohydrate loading, the old technique discovered by two Swedish researchers, Saltin and Hermansen, in 1960s.
The researchers discovered that muscle glycogen concentration could be doubled in the six days before the competition following this diet:
- three days of low carb menu (a nutritional plan very poor in carbohydrates, i.e. without pasta, rice, bread, potatoes, legumes, fruits etc.);
- three days of high carbohydrate diet, the so-called carbohydrate loading (a nutritional plan very rich in carbohydrates).
This diet causes a lot of problems: the first three days are very hard and there may be symptoms similar to depression due to low glucose delivery to brain, and the benefits are few.
Moreover, with the current training techniques, the type and amount of work done, we can indeed obtain high levels of glycogen: above 2.5 g/kg of body weight.
The “corrent” carbohydrate loading
If we compete on Sunday, a possible training/nutritional plan to obtain supercompensation of glycogen stores can be the following:
- Wednesday, namely four days before the competition, moderate training and then dinner without carbohydrates;
- from Thursday on, namely the three days before the competition, hyperglucidic diet and light trainings.
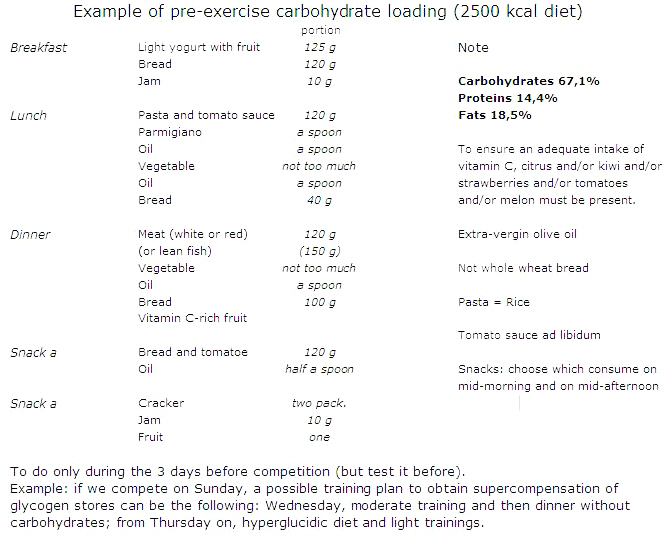
The amount of dietary carbohydrates needed to recover glycogen stores or to promote glycogen loading depends on the duration and intensity of the training programme, and they span from 5 to 12 g/kg of body weight/d, depending on the athlete and his activity. With higher carbohydrate intake you can achieve higher glycogen stores but this does not always results in better performance. Moreover, it should be noted that glycogen storage is associated with weight gain due to water retention (approximately 3 g per gram of glycogen), and this may not be desirable in some sports.
References
- Burke L.M., Hawley J.A., Wong S.H.S., & Jeukendrup A. Carbohydrates for training and competition. J Sport Sci 2011;29:Sup1,S17-S27. doi:10.1080/02640414.2011.585473
- Hargreaves M., Hawley J.A., & Jeukendrup A.E. Pre-exercise carbohydrate and fat ingestion: effects on metabolism and performance. J Sport Sci 2004;22:31-38. doi10.1080/0264041031000140536
- Jeukendrup A.E., C. Killer S.C. The myths surrounding pre-exercise carbohydrate feeding. Ann Nutr Metab 2010;57(suppl 2):18-25. doi:10.1159/000322698
- Moseley L., Lancaster G.I, Jeukendrup A.E. Effects of timing of pre-exercise ingestion of carbohydrate on subsequent metabolism and cycling performance. Eur J Appl Physiol 2003;88:453-458. doi:10.1007/s00421-002-0728-8