The Cori cycle was discovered in the 1930s and 1940s by Carl Ferdinand Cori and Gerty Theresa Radnitz, a husband-and-wife research team. They demonstrated the existence of a metabolic cooperation between skeletal muscle, operating under hypoxic conditions, and the liver.[1]
The cycle enables the conversion of lactate, the conjugate base of lactic acid and its predominant form at physiological pH, back into glucose, thereby ensuring a continuous supply of this monosaccharide to peripheral tissues. Its physiological importance is underscored by the fact that it accounts for up approximately 36% of plasma glucose turnover.[2][3]
From a biochemical perspective, the Cori cycle connects gluconeogenesis and anaerobic glycolysis, using different tissues to compartmentalize these opposing metabolic pathways.[4]
This metabolic cooperation also occurs between the liver and other extrahepatic tissues. Indeed, like the glucose-alanine cycle, the Cori cycle is active between the liver and all tissues that do not fully oxidize glucose to CO2 and H2O.[5]
Contents
- Pathway
- Energy cost
- Is the Cori cycle a futile cycle?
- Similarities with the glucose-alanine cycle
- Differences from the glucose-alanine cycle
- References
Pathway
The steps of the Cori cycle are typically analyzed by considering the lactate produced by red blood cells and muscle fibers.
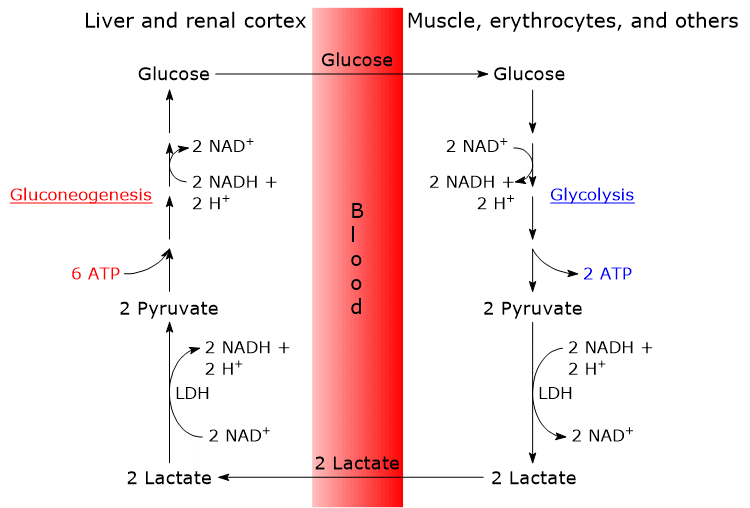
- The cycle begins with the conversion of glucose to lactate via anaerobic glycolysis, followed by the action of lactate dehydrogenase (LDH; EC 1.1.1.27), which catalyzes the reduction of pyruvate, the conjugate base of pyruvic acid.[6]
- Lactate then diffuses out of the cell and into the bloodstream, through which it is transported to the liver, its primary site of utilization, and to the renal cortex, particularly the proximal tubules, another site where gluconeogenesis occurs.[6]
- In both the liver and renal cortex, lactate is oxidized back to pyruvate in a reaction also catalyzed by lactate dehydrogenase. Pyruvate is subsequently converted into glucose via the gluconeogenic pathway.[7]
- Finally, glucose enters the bloodstream and is delivered to red blood cells and muscle fibers, thereby completing the cycle.[8]
Red blood cells
Mature red blood cells, lacking a nucleus, ribosomes, and mitochondria, are smaller than most other cells. Their small size allows them to pass through narrow capillaries, but the absence of mitochondria makes them completely dependent on anaerobic glycolysis for ATP production. As a result, these cells continuously produce lactic acid.[8]
The availability of NAD+ is essential not only for glycolysis to proceed but also to sustain its rate. The oxidized form of this coenzyme is required for the oxidation of glyceraldehyde 3-phosphate to 1,3-bisphosphoglycerate, a reaction catalyzed by glyceraldehyde 3-phosphate dehydrogenase (EC 1.2.1.12).[3]
Glyceraldehyde 3-phosphate + NAD+ → 1,3-Bisphosphoglycerate + NADH + H+
The accumulation of NADH is prevented by the reduction of pyruvate to lactate, in a reaction catalyzed by lactate dehydrogenase, in which NADH acts as the reducing agent, being oxidized back to NAD+.[9][10]
Muscle fibers
Fast-twitch muscle fibers contain relatively few mitochondria and, under hypoxic conditions, such as during intense exercise, produce significant amounts of lactic acid.[3] In these conditions:
- the rate of pyruvate production via glycolysis exceeds the rate of its oxidation through the citric acid cycle, so that less than 10% of the pyruvate enters the cycle;[7]
- the rate of oxygen uptake by the cells is insufficient to support the aerobic oxidation of all the NADH produced.[11]
As a result, anaerobic glycolysis leads to the production of 2 ATP per molecule of glucose (or 3 ATP if the glucose is derived from muscle glycogen), a much lower yield compared to the 29–30 ATP generated through complete oxidation of glucose.[10]
However, the rate of ATP production via anaerobic glycolysis is higher than that of complete aerobic oxidation.[8]
Finally, as in red blood cells, the reaction catalyzed by lactate dehydrogenase, which regenerates NAD+, enables glycolysis to continue, though it results in lactate production.[3]
Lactate
Lactic acid is a metabolic end product that must be converted back into pyruvate in order to be reutilized.[7]
The plasma membrane of most cells is freely permeable to both pyruvate and lactate, allowing them to enter the bloodstream.[12]
In skeletal muscle, for example, the amount of lactate that leaves the cell is greater than that of pyruvate. This is due to the high NADH/NAD+ ratio in the cytosol and the catalytic properties of the lactate dehydrogenase isozyme expressed in muscle fibers.[13]
Once in the bloodstream, lactate reaches, among other tissues, the liver and the renal cortex, where it is oxidized back to pyruvate by tissue-specific LDH isozymes.[14]
In the hepatocyte, this oxidation is favored by the low NADH/NAD+ ratio in the cytosol. The resulting pyruvate then enters gluconeogenesis, the next step in the Cori cycle, and is converted into glucose.[7]
Glucose subsequently enters the bloodstream and is delivered to muscle fibers and red blood cells, thus completing the cycle. Naturally, the monosaccharide also reaches all other tissues and cells that require it.[15]
Energy cost
The Cori cycle results in a net consumption of 4 ATP per cycle.
The gluconeogenic phase of the cycle consumes 2 GTP and 4 ATP per molecule of glucose synthesized, totaling 6 high-energy phosphate bonds.
>The ATP- (or GTP-) consuming reactions are catalyzed by the following enzymes:
- pyruvate carboxylase (EC 6.4.1.1): 1 ATP;
- phosphoenolpyruvate carboxykinase (EC 4.1.1.32): 1 GTP;
- glyceraldehyde 3-phosphate dehydrogenase (EC 1.2.1.12): 1 ATP.
Since two molecules of lactate are needed to synthesize one molecule of glucose, the net energy cost is:
2 x 3 = 6 high energy bonds per molecule of glucose.
In contrast, the glycolytic phase of the cycle yields only 2 ATP per molecule of glucose.
Therefore, more energy is required to convert lactate back into glucose than is gained from the initial anaerobic glycolysis in extrahepatic tissues. This explains why the Cori cycle cannot be sustained indefinitely.[15]
Is the Cori cycle a futile cycle?
The continuous breakdown and resynthesis of glucose, characteristic of the Cori cycle, might initially appear to be a waste of energy. In reality, however, this cycle enables many extrahepatic cells to function effectively, at the energetic expense of the liver and renal cortex.
Therefore, it is more accurate to describe the Cori cycle as a substrate cycle, rather than a futile cycle.[8]
Below are some illustrative examples.
- The Cori cycle contributes to the disposal of lactic acid produced continuously by red blood cells.[3]
- During intense exercise, anaerobic glycolysis is a major source of ATP for muscle fibers. This process, however, could lead to the accumulation of lactate and a subsequent decrease in intracellular pH.
This accumulation is largely prevented by the Cori cycle, in which gluconeogenic tissues (mainly liver and renal cortex) bear the energy cost of removing excess muscle lactate.[16]
Furthermore, the oxygen debt incurred after intense exercise is largely due to the increased oxygen demand of hepatocytes, where the oxidation of fatty acids, their primary fuel, supplies the ATP needed for gluconeogenesis.[15][17] - In conditions such as trauma, sepsis, burns, or major surgery, intense cell proliferation occurs in the wound (a hypoxic environment) and in the bone marrow. This leads to increased lactic acid production, a greater flux through the Cori cycle, and thus increased ATP consumption by the liver.[18] As previously mentioned, this demand is met by enhanced fatty acid oxidation.
A similar situation seems to occur in cancer patients experiencing progressive weight loss.[19] - The Cori cycle also plays an important role during overnight fasting and starvation, helping maintain blood glucose levels.[5][17]
Similarities with the glucose-alanine cycle
- These two metabolic pathways, which operate across different organs via the bloodstream, contribute to maintaining a continuous supply of glucose to peripheral tissues.
- In both cycles, entry into gluconeogenesis involves the conversion of alanine or lactate into pyruvate.
- Finally, in both cycles, the glucose produced is delivered to peripheral tissues, where it is metabolized via glycolysis, regenerating pyruvate.[15]
Differences from the glucose-alanine cycle
The main difference between the two cycles lies in the three-carbon intermediate that is recycled:
- in the Cori cycle, carbon is returned to the liver as pyruvate.
- in the glucose-alanine cycle, it is returned as alanine.[20]
Additionally, the metabolic fate of NADH also differs:
- in the Cori cycle, NADH acts as a reducing agent in the reaction catalyzed by lactate dehydrogenase;
- in the glucose-alanine cycle, the electrons from NADH are used to synthesize ATP in the mitochondria.[15]
As a result, another key difference is that the glucose-alanine cycle requires oxygen, whereas the Cori cycle can function under anaerobic conditions.[3]
| Feature | Cori Cycle | Glucose-Alanine Cycle |
|---|---|---|
| Carbon carrier | Lactate | Alanine |
| Intermediate returned to the liver | Pyruvate (via lactate) | Pyruvate (via alanine) |
| NADH fate | Reoxidized to NAD⁺ by lactate dehydrogenase | Used in mitochondria to generate ATP |
| Oxygen requirement | No | Yes |
| Main context of activation | Intense exercise, red blood cells | Muscle protein catabolism, prolonged fasting |
| Additional function | Prevents lactate accumulation and acidosis | Transports nitrogen for urea synthesis |
References
- ^ American Chemical Society National Historic Chemical Landmarks. Carl and Gerty Cori and Carbohydrate Metabolism. https://www.acs.org/education/whatischemistry/landmarks/carbohydratemetabolism.html. Accessed June 27, 2025.
- ^ Katz J., Tayek J.A. Gluconeogenesis and the Cori cycle in 12-, 20-, and 40-h-fasted humans. Am J Physiol 1998;275(3):E537-42. doi:10.1152/ajpendo.1998.275.3.E537
- ^ a b c d e f Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 6th Edition. W.H. Freeman and Company, 2012.
- ^ Rodwell V.W., Bender D.A., Botham K.M., Kennelly P.J., Weil P.A. Harper’s Illustrated Biochemistry. 31st Edition. McGraw-Hill, 2018.
- ^ a b Rui L. Energy metabolism in the liver. Compr Physiol 2014;4(1):177-97. doi:10.1002/cphy.c130024
- ^ a b Stipanuk M.H., Caudill M.A. Biochemical, physiological, and molecular aspects of human nutrition. 3rd Edition. Elsevier health sciences, 2012.
- ^ a b c d Berg J.M., Tymoczko J.L., and Stryer L. Biochemistry. 5th Edition. W. H. Freeman and Company, 2002.
- ^ a b c d Rosenthal M.D., Glew R.H. Medical biochemistry – Human metabolism in health and disease. John Wiley J. & Sons, Inc., Publication, 2009.
- ^ Spinelli S., Marino A., Morabito R., Remigante A. Interplay between metabolic pathways and increased oxidative stress in human red blood cells. Cells 2024;13(23):2026. doi:10.3390/cells13232026
- ^ a b Garrett R.H., Grisham C.M. Biochemistry. 4th Edition. Brooks/Cole, Cengage Learning, 2010.
- ^ Rich P.R. The molecular machinery of Keilin’s respiratory chain. Biochem Soc Trans 2003;31(Pt 6):1095-105. doi:10.1042/bst0311095
- ^ Halestrap A.P. The monocarboxylate transporter family — Structure and functional characterization. IUBMB Life 2012;64(1):1-9. doi:10.1002/iub.573
- ^ Gleeson T.T. Post-exercise lactate metabolism: a comparative review of sites, pathways, and regulation. Annu Rev Physiol 1996;58:565-81. doi:10.1146/annurev.ph.58.030196.003025
- ^ Gladden L.B. Lactate metabolism: a new paradigm for the third millennium. J Physiol 2004;558(Pt 1):5-30. doi:10.1113/jphysiol.2003.058701
- ^ a b c d e Voet D. and Voet J.D. Biochemistry. 4th Edition. John Wiley J. & Sons, Inc. 2011.
- ^ van Hall G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol (Oxf) 2010;199(4):499-508. doi:10.1111/j.1748-1716.2010.02122.x
- ^ a b Moran L.A., Horton H.R., Scrimgeour K.G., Perry M.D. Principles of Biochemistry. 5th Edition. Pearson, 2012.
- ^ Liu S., Yang T., Jiang Q., Zhang L., Shi X., Liu X., Li X. Lactate and lactylation in sepsis: a comprehensive review. J Inflamm Res 2024;17:4405-4417. doi:10.2147/JIR.S459185
- ^ Li X., Yang Y., Zhang B., Lin X., Fu X., An Y., Zou Y., Wang J.X., Wang Z., Yu T. Lactate metabolism in human health and disease. Signal Transduct Target Ther 2022;7(1):305. doi:10.1038/s41392-022-01151-3
- ^ Felig P., Pozefsk T., Marlis E., Cahill G.F. Alanine: key role in gluconeogenesis. Science 1970;167(3920):1003-1004. doi:10.1126/science.167.3920.1003