Linoleic acid (LA) is a fatty acid, therefore a carboxylic acid, with a straight carbon-chain of eighteen carbon atoms and two double bonds.
Its name derives from the Latin linon, meaning flax, plus oleic, meaning oil.
It was isolated by Sacc F. in 1844 from linseed oil, the structure was clarified by Hilditch T.P. et al. in 1939, and was synthesized by Raphael R.A. and Sondheimer F. in 1950.[9][19][21]
Many animals, including humans, can’t synthesize it. For these animals, linoleic acid and alpha-linolenic acid (ALA), which is the precursor of omega-3 polyunsaturated fatty acids or omega-3 fatty acids, are essential fatty acids, that is, fatty acids that must be obtained from the diet.[24] Moreover, in the absence of dietary LA, the other omega-6 PUFAs gamma-linolenic acid, dihomo-gamma-linolenic acid, arachidonic acid (ARA), and adrenic acid become essential, too, as it is their precursor. For this reason, they are defined as conditionally essential fatty acids.[3]
Like the other fatty acids, it is rarely found in free form; most commonly it is bound through ester bonds to organic molecules such as sterols, glycerol, and glycerol 3-phosphate to form more complex lipids, such as sterol esters, for example cholesterol esters, triglycerides, and phospholipids.
In humans, it is abundantly found in adipose tissue and plasma lipoproteins.
It performs many functions both directly and indirectly through its metabolites. It may be used for energy, is a structural component of cell membranes, and is involved in the maintenance of transepidermal water barrier.[27] It serves as precursor for the synthesis of omega-6 polyunsaturated fatty acids. Furthermore, it may be desaturated to alpha-linolenic acid in vascular terrestrial plants and phytoplankton. And, like ARA and docosahexaenoic acid (DHA), an omega-3 fatty acid, linoleic acid serves as precursor for the synthesis of bioactive lipid mediators. Finally, it has been shown that dietary LA intake is inversely related to the risk of coronary heart disease when it replaces either saturated fatty acids or carbohydrates.[7]
It is the most abundant PUFA in the Western diet, and is the only one whose intake is significantly increased, having reached, on average, over 7 percent of daily energy intake, whereas the intake of the other omega-6 and omega-3 fatty acids has remained relatively constant since the early 1900s, corresponding to less than 1 percent of the daily energy intake.[2][25] The high LA intake has shifted the omega-6 to omega-3 ratio from 4:1 to about 16:1.[23]
Linoleic acid deficiency results in retardation in growth and wound healing, dermatitis, reproductive problems, fatty liver and polydipsia. However, its deficiency is extremely rare due to its abundance in the Western diet, and consequently in breast milk, and to its presence in baby formulas.[3]
The main dietary sources are soybean, corn, sunflower, canola and safflower oils.[3][27]
Contents
Properties
Linoleic acid has a molecular weight of 280.447 g/mol, molecular formula C18H32O2, condensed formula HOOC(CH2)7CH=CHCH2CH=CH(CH2)4CH3, IUPAC name (9Z,12Z)-octadeca-9,12-dienoic acid, and shorthand notation 18:2n-6.[18]
Its conjugate base is called linoleate, has condensed formula C18H31O2–, and is the form in which, at physiological pH, the free fatty acid is present in biological systems.
As its carbon-chain has two cis double bonds, at positions 9 and 12, linoleic acid belongs to the group of unsaturated fatty acids. And, since the first double bond, with respect to the methyl end, is located at position 6, it also belongs to the group of omega-6 polyunsaturated fatty acids (PUFAs) or omega-6 fatty acids.[1]
In purified form it is a colorless to straw colored liquid, with a melting point is 5 °C (23 °F; 268.15 K), and a boiling point of 230 °C (446 °F; 503.15 K) at 16 mm Hg.[18]
Its CAS registry number, PubChem compound identifier, and European Community (EC) Number are 60-33-3, 5280450, and 200-470-9, respectively.[18]
Synthesis
Linoleic acid is synthesized from oleic acid, an unsaturated fatty acid with a chain of 18 carbon atoms and a cis double bond at position 9 with respect to the methyl end, therefore an omega-9 fatty acid.
The reaction, catalyzed by delta-12 desaturase (EC 1.14.19.6), introduces a second cis double bond between the 12th and 13th carbon atom, and is the critical step in PUFA biosynthesis.[15]
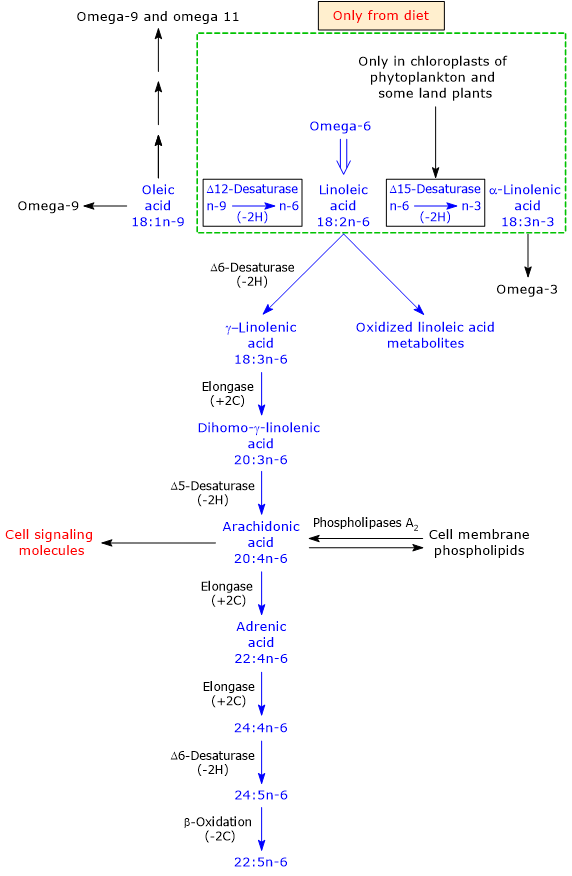
It should be underscored that delta-12 desaturase catalyzes the shift from an omega-9 fatty acid to an omega-6 fatty acid.
It was long thought that this enzyme was present only in Bacteria, Protozoa and Plants, and that only these organisms were able to synthesize linoleic acid.[5] However, in 1961 it was shown that an insect, the American cockroach Periplaneta americana, was able to synthesize linoleic acid.[14] Subsequently, LA synthesis was detected in other insects and other invertebrates, including several Nematodes, Crustaceans and Acari.[15]
Tissue distribution
Linoleic acid, with palmitic acid and oleic acid, is one of the most abundant fatty acids in triglycerides of adipose tissue and plasma lipoproteins, where its concentration reflects dietary intake.[3] In the USA, the large increase in dietary LA intake has been accompanied by an increase in its content in adipose tissue, which has been estimated >135 percent.[8]
Due to its abundance and susceptibility to oxidation, linoleic acid is the fatty acid that is most commonly oxidized in LDLs.[11] And oxidation of LDL lipids is a risk factor for coronary heart disease.[16]
Although essential for fetal neurodevelopment, it is present in low concentrations in the brain, less than 2 percent of total fatty acids, much lower than palmitic acid, stearic acid, oleic acid, DHA and ARA, which account for over 84 percent of brain fatty acids.[25][26]
It is the most abundant PUFA in the epidermis.[3]
Metabolites
Linoleic acid is the precursor of all omega-6 fatty acids.
It can be desaturated to alpha-linolenic acid, the precursor of omega-3 PUFAs. The reaction is catalyzed by delta-15 desaturase or omega-3 desaturase or fatty acid desaturase 3 (EC 1.14.19.25), that is present only in the plastids and in the endoplasmic reticulum of phytoplankton and vascular terrestrial plants.[1][3] It should be underscored that delta-15 desaturase catalyzes the shift from an omega-6 fatty acid to an omega-3 fatty acid.
It is the precursor of bioactive lipid mediators with autocrine and paracrine effect, which are involved in the regulation of many cellular processes.[27] Similarly to arachidonic acid and docosahexaenoic acid, when released from membrane phospholipids, it may be oxidized in reactions catalyzed by enzymes such as 12-lipoxygenase (EC 1.13.11.31), 15-lipoxygenase (EC 1.13.11.33), cyclooxygenases (EC 1.14.99.1), soluble epoxy hydrolase (EC 3.3.2.10), and by enzymes of the cytochrome P450 family, namely, the same enzymes involved in the metabolism of ARA and DHA.[22] It may be also oxidized by free radicals, too.[25] The mediators produced, such as epoxy- and mono-, di-, and trihydroxy derivatives, which are called oxidized linoleic acid metabolites (OXLAMs), are involved in cell signaling and regulation of pain and inflammation.[22]
Because linoleic acid can be converted to arachidonic acid, which is the precursor of bioactive lipid mediators, some with anti-inflammatory activities or able to promote the resolution of inflammatory injuries, others with pro-inflammatory activities, it was hypothesized that tissue levels of ARA could be reduced by reducing LA intake.
However, at least in subjects who consume a typical Western diet, this does not seem to occur, as LA conversion to ARA is very modest, between 0.3 percent and 0.6 percent, similarly to what occurs in the conversion of ALA to DHA.[20] Furthermore, it has been suggested that the profile of lipid mediators, as well as inflammatory response to a diet rich in linoleic acid are influenced by the FADS1 genotype, where FADS1 gene encodes for delta-5 desaturase (EC 1.14.19.44), the enzyme that catalyzes the second desaturation reaction of arachidonic acid synthesis pathway, that is, the desaturation of dihomo-gamma-linolenic acid to ARA.[13]
Role
For most animals, including humans, linoleic acid is an essential fatty acid, and plays important roles acting either directly or through its metabolites.
- It can be used as an energy source.[27] For example, it enters the brain at a rate comparable to that of DHA, ARA and other fatty acids. ARA and DHA are mostly incorporated into membrane phospholipids, whereas almost 60 percent of linoleic acid is oxidized by the beta-oxidation pathway. Furthermore, part of the acetate produced is used for cholesterol synthesis within the brain.[4]
- As a component of membrane phospholipids, it is involved in the maintenance of membrane fluidity.[27]
- As a component of membrane sphingolipids, it is involved in the formation and maintenance of the water permeability barrier of the skin.[28]
- It can bind to and activate PPARalpha.[8]
This transcription factor plays a major role in metabolic regulation. For example, it inhibits the transcription of genes coding for enzymes involved in lipogenesis, and activates the transcription of gene coding for enzymes involved in lipolysis and in mitochondrial and lysosomal beta-oxidation.[6] In this way, linoleic acid may reduce plasma levels of total and LDL cholesterol, thus contributing to the reduction of cardiovascular risk.[7]
Food sources
During the twentieth century the consumption of vegetable oils rich in linoleic acid is increased 20-fold in the United States.[2][17] This increase was due to:
- the development of cultivars that produce seeds with high LA content, particularly soy and corn;[2]
- an increased commercial availability of the derived oils;
- dietary recommendations to increase its consumption as an aid to lower blood cholesterol levels, recommendations resulting from studies by the American physiologist Ancel Keys in the mid-1950s.[12]
This has led LA intake to the current levels of about 6-7 percent of total dietary energy, compared to pre-1930s values, that ranged between 1 to 2 percent of total dietary energy.[25]
In the US diet, soybean oil is the major LA dietary source, accounting for about 45 percent of its dietary intake.[27]
Other foods rich in linoleic acid are corn, sunflower, safflower, and canola oils and the corresponding seeds, as well as walnuts, peanuts, cottonseed and sesame seeds.[3]
Animals fed with diets rich in linoleic acid can also be a good source.
It is the most abundant PUFA in foods. The highest values are found again in soybean oil, where it accounts for up to 88 percent of total PUFAs, and exceeds 70 percent of the total PUFAs in the most commonly consumed foods. For example, it ranges between 75 and 85 percent of the total PUFAs in pork, beef and chicken meats, and exceeds 80 percent in eggs. Moreover, it is the major PUFA in vegetables, fruits, and grains, that is, foods with a low fat content. The exception is beans where it accounts for 40-50 percent of the total PUFAs.[2][27]
New cultivars
In nature, linoleic acid is synthesized in concentrations comparable to those of alpha-linolenic acid.
Although in the 1930s cultivars have been selected that produce seeds with high LA content, in recent years this trend is changing as new cultivars have been selected to produce seeds with a lower LA content.[25] Moreover, for the most part linoleic acid has been replaced by oleic acid.[10]
This has allowed to obtain not only sunflower and safflower oils with high oleic acid content instead of high LA content, but also soybeans with high oleic acid content. And as the new cultivars will replace the traditional cultivars, a reduction in the consumption of linoleic acid in favor of oleic acid will presumably occur. This could shift the levels of LA intake back to levels comparable to those at the beginning of the 1900s. This transition could bring health benefits as the consumption of oleic acid is associated with improvements in HDL cholesterol levels. It should be underscored that a good dietary intake of oleic acid, in the form of extra virgin olive oil, is a feature of the mediterranean diet.[10]
References
- ^ a b Akoh C.C. and Min D.B. Food lipids: chemistry, nutrition, and biotechnology. 3th Edition. CRC Press, Taylor & Francis Group, 2008
- ^ a b c d Blasbalg T. L., Hibbeln J. R., Ramsden C. E., Majchrzak S. F. & Rawlings R. R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr 2011;93(5):950-962. doi:10.3945/ajcn.110.006643
- ^ a b c d e f g Chow Ching K. Fatty acids in foods and their health implication. 3th Edition. CRC Press, Taylor & Francis Group, 2008
- ^ DeMar J.C. Jr, Lee H.J., Ma K., Chang L., Bell J.M., Rapoport S.I., Bazinet R.P. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim Biophys Acta 2006;1761(9):1050-9. doi:10.1016/j.bbalip.2006.06.006
- ^ De Renobales M., Ryan R.O., Heisler C.R., McLean D.L., Blomquist G.J. Linoleic acid biosynthesis in the pea aphid, Acyrthosiphon pisum (Harris). Arch Insect Biochem Physiol 1986:3(2);193-203.
- ^ Desvergne B., Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 1999;20(5):649-88. doi:10.1210/edrv.20.5.0380
- ^ a b Farvid M.S., Ding M., Pan A., Sun Q., Chiuve S.E., Steffen L.M., Willett W.C., Hu F.B. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation 2014;130:1568-1578. doi:10.1161/CIRCULATIONAHA.114.010236
- ^ a b Guyenet S.J., Carlson S.E. Increase in adipose tissue linoleic acid of US adults in the last half century. Adv Nutr 2015;6(6):660-664. doi:https://DOI.org/10.3945/an.115.009944
- ^ Hilditch T.P. and Jasperson H. The constitution of the linoleic acid of seed fats. J Soc Chem Ind 1939;58(26). doi:10.1002/jctb.5000582613
- ^ a b Jandacek R.J. Linoleic acid: a nutritional quandary. Healthcare 2017;5(2):25. doi:10.3390/healthcare5020025
- ^ Jira W., Spiteller G., Carson W., Schramm A. Strong increase in hydroxy fatty acids derived from linoleic acid in human low density lipoproteins of atherosclerotic patients. Chem Phys Lipids 1998;91(1):1-11. doi:10.1016/s0009-3084(97)00095-9
- ^ Keys A., Anderson J.T., Grande F. Prediction of serum-cholesterol responses of man to changes in fats in the diet. Lancet 1957;273(7003):959-66. doi:10.1016/s0140-6736(57)91998-0
- ^ Lankinen M.A., Fauland A., Shimizu B., Agren J., Wheelock C.E., Laakso M., Schwab U., and Pihlajamaki J. Inflammatory response to dietary linoleic acid depends on FADS1 genotype. Am J Clin Nutr 2019;109:165-175. doi:10.1093/ajcn/nqy287
- ^ Louloudes S.J., Kaplanis J.N., Robbins W. E., Monroe R.E. Lipogenesis from C14-acetate by the American cockroach. Ann Entomol Soc Am 1961;54(1):99-103. doi:10.1093/aesa/54.1.99
- ^ a b Malcicka M., Visser B., Ellers J. An evolutionary perspective on linoleic acid synthesis in animals. Evol Biol 2018;45:15-26. doi:10.1007/s11692-017-9436-5
- ^ Meisinger C., Baumert J., Khuseyinova N., Loewel H., Koenig W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation 2005;112(5):651-7. doi:10.1161/CIRCULATIONAHA.104.529297
- ^ Micha R., Khatibzadeh S., Shi P., Fahimi S., Lim S., Andrews K.G., Engell R.E., Powles J., Ezzati M., Mozaffarian D.; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group NutriCoDE. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys. BMJ 2014;348:g2272. doi:10.1136/bmj.g2272
- ^ a b c National Center for Biotechnology Information. PubChem Compound Summary for CID 5280450, Linoleic Acid. https://pubchem.ncbi.nlm.nih.gov/compound/Linoleic-Acid. Accessed Jan. 19, 2024
- ^ Raphael R.A., Sondheimer F. The synthesis of long-chain aliphatic acids from acetylenic compounds. Part III. The synthesis of linoleic acid. J Chem Soc 1950:2100-2103. doi:10.1039/jr9500002100
- ^ Rett B.S. and Whelan J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: a systematic review. Nutr Metab (Lond) 2011;8:36. doi:10.1186/1743-7075-8-36
- ^ Sacc F. Ueber das Leinöl, seine physicalischen und chemischen Eigenscharften und seine Oxydationsproducte”. Liebig Annalen 1844;51;213-230.
- ^ a b Schuster S. Johnson C.D, Hennebelle M., Holtmann T., Taha A.Y., Kirpich I.A., Eguchi A., Ramsden C.E., Papouchado B.G., McClain C.J., Feldstein A.E. Oxidized linoleic acid metabolites induce liver mitochondrial dysfunction, apoptosis and NLRP3 activation in mice. J Lipid Res 2018;59(9):1597-1609. doi:10.1194/jlr.M083741
- ^ Simopoulos A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med 2008;233(6):6746-88. doi:10.1016/S0753-3322(02)00253-6
- ^ Smith W., Mukhopadhyay R. Essential fatty acids: the work of George and Mildred Burr. J Biol Chem 2012;287(42):35439-35441. doi:10.1074/jbc.O112.000005
- ^ a b c d e Taha A.Y. Linoleic acid-good or bad for the brain? NPJ Sci Food 2020;4:1. doi:10.1038/s41538-019-0061-9
- ^ Taha A.Y., Cheon Y., Ma K., Rapoport S.I., Rao JS.. Altered fatty acid concentrations in prefrontal cortex of schizophrenic patients. J Psychiatr Res 2013;47(5):636-43. doi:10.1016/j.jpsychires.2013.01.016
- ^ a b c d e f g Whelan J. and Fritsche K. Linoleic acid. Adv Nutr 2013;4(3): 311-312. doi:10.3945/an.113.003772
- ^ Ziboh V.A. Prostaglandins, leukotrienes, and hydroxy fatty acids in epidermis. Semin Dermatol 1992;11(2):114-20