Lipids, together with carbohydrates, proteins, and nucleic acids, are one of the four main classes of organic molecules found in living organisms.
They constitute a broad class that includes molecules with diverse structures, differing in the atoms that compose them, the types of covalent bonds they contain, and the presence or absence of cyclic structures. Examples include fatty acids, triglycerides, phospholipids, glycolipids, sterols such as cholesterol, terpenes such as carotenoids, and vitamins A, D, E, and K, collectively known as fat-soluble vitamins. Different lipids exhibit different properties; for example, some are soluble in non-polar solvents, whereas others are amphipathic and partially soluble in certain polar solvents.
Along with carbohydrates and proteins, lipids are one of the three macronutrients and perform a wide range of functions in the body. These include serving as energy stores, acting as electrical and thermal insulators, forming structural components of biological membranes, and participating in the regulation of numerous cellular processes by functioning as second messengers, hormones, and membrane receptors. Moreover, lipids facilitate the digestive process.
Lipid digestion is complicated by the insolubility of many lipids in the aqueous environment of the intestine and therefore requires, in addition to digestive enzymes, the action of bile salts. Lipid absorption, or, more precisely, the absorption of the products of lipid digestion, is also influenced by the solubility of the released molecules, as are their subsequent transport, storage, and utilization.
Summary: Key Points
- Chemical nature: hydrophobic and amphipathic molecules, soluble in non-polar solvents.
- Classification: divided into simple (triglycerides), complex (phospholipids), and derivatives (fatty acids and sterols).
- Functions: energy reserve (9 kcal/g), membrane structure, thermal insulation, and hormonal signaling.
- Essential nutrients: includes fat-soluble vitamins (A, D, E, K) and essential fatty acids (Omega-3 and Omega-6).
- Digestion and absorption: requires bile salts and the formation of mixed micelles for absorption.
Contents
- Structure
- Molecular weight
- Solubility
- Classification of lipids
- Functions of lipids
- Digestion
- Intestinal absorption
- References
Structure
The simplest lipids are fatty acids, which are carboxylic acids with a hydrocarbon chain of variable length. In most cases, the hydrocarbon chain is straight. If there are no double (or rarely triple) bonds in the chain, fatty acids are called saturated fatty acids, whereas if one or more are present, they are called unsaturated fatty acids. When the hydrocarbon chain contains two or more double bonds, fatty acids are referred to as polyunsaturated.
Examples of more complex lipids include triglycerides, phospholipids, and sterols. Triglycerides consist of three fatty acids attached to a glycerol backbone. Phospholipids consist of two fatty acids and a phosphate group attached to a glycerol backbone. In turn, the phosphate group can be esterified to an organic molecule such as serine, inositol, choline, or ethanolamine. Sterols differ from other lipids in that they consist of interconnected rings of carbon atoms, with side chains containing oxygen, carbon, and hydrogen.
Molecular weight
Unlike many polysaccharides, proteins, and nucleic acids, lipids are not polymers but relatively small molecules whose molecular weights vary greatly, typically ranging from about 1,000 – 1,500daltons (Da). For example, acetic acid, the smallest fatty acid, has a molecular weight of 60.05 Da, while lignoceric acid, one of the very long-chain fatty acids, has a molecular weight of 368.37 Da. By contrast, the molecular weights of proteins range from approximately 5,000–10,000 Da for the smallest proteins to several million daltons for the largest.
Solubility
Lipids are generally considered to be insoluble in polar solvents and soluble in non-polar solvents, and this is true for many of them. However, the presence of atoms such as oxygen, and especially phosphorus, increases the solubility of lipid molecules, or at least of parts of them, in polar solvents. For example, the solubility of straight-chain saturated fatty acids in polar solvents is a function of the length of the hydrocarbon chain, which is non-polar, in contrast to the polar carboxyl group. Therefore, while butyric acid, one of the short-chain fatty acids, is soluble in polar solvents, the solubility of caproic, caprylic, capric, and lauric acids, whose hydrocarbon chains contain 6, 8, 10, and 12 carbon atoms, respectively, gradually decreases as chain length increases.
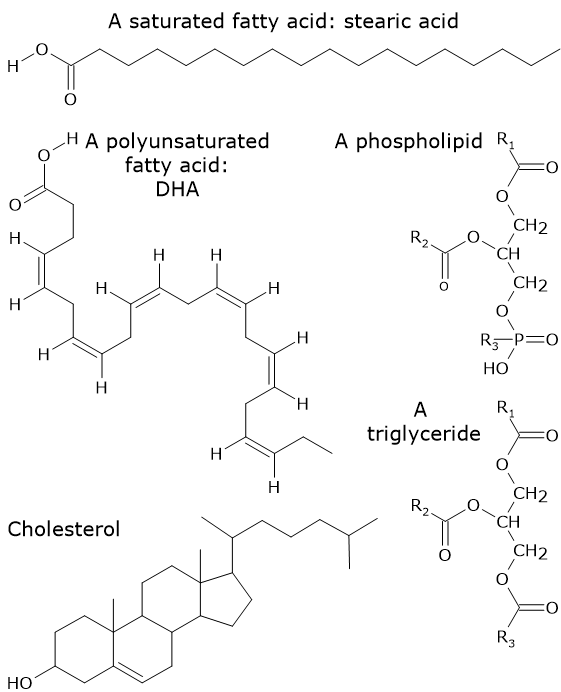
Saturated fatty acids with a chain length greater than 16–18 carbon atoms, namely palmitic acid and stearic acid and, consequently, arachidic, behenic, and lignoceric acids are insoluble in polar solvents. Other examples of hydrophobic lipids include triglycerides containing long- and very-long-chain fatty acids, as well as cholesterol esters.
Finally, it should be emphasized that many lipids are amphipathic molecules, that is, molecules containing both polar and non-polar regions. Examples include fatty acids, phospholipids, glycolipids, and cholesterol.
Classification of lipids
As with carbohydrate and protein classification, there are several ways to classify lipids. They may be classified according to their physical properties at room temperature, thus as solids (fats) or liquids (oils), or on the basis of polarity. However, the preferred classification is based on their chemical structure, according to which lipids are divided into three groups: simple lipids, complex lipids, and derived lipids.
Simple lipids
Simple lipids consist of two types of structural moieties and include:
- esters of glycerol and fatty acids, such as triglycerides, monoacylglycerols, and diacylglycerols;
- esters of cholesterol and fatty acids;
- waxes, which are esters of long-chain alcohols and fatty acids, including esters of vitamins A and D;
- ceramides, that is, amides of fatty acids with long-chain di- or trihydroxy bases containing 12–22 carbon atoms in the carbon chain; an example is sphingosine.
Complex lipids
Unlike simple lipids, complex lipids consist of more than two types of structural moieties and include, among others:
- phospholipids, that is, glycerol esters of fatty acids, phosphoric acid, and other groups containing nitrogen;
- phosphatidic acid, that is, diacylglycerol esterified with phosphoric acid;
- phosphatidylcholine (also called lecithin), phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol, that is, phosphatidic acid linked to choline, ethanolamine, serine, and inositol, respectively;
- sphingolipids, which are derivatives of ceramides;
- sphingomyelin, that is, ceramide phosphorylcholine;
- cerebrosides, which are ceramide monohexosides, that is, ceramide linked to a single carbohydrate moiety at the terminal hydroxyl group of the base;
- ceramide di- and polyhexosides, that is, ceramides linked to a disaccharide or to a tri- or oligosaccharide, respectively;
- cerebroside sulfates, that is, ceramide monohexosides esterified with a sulfate group.
Derived lipids
Derived lipids are the building blocks of simple and complex lipids and may occur as such or be released from the other two major lipid groups. They include fatty acids and alcohols, vitamins A, D, E, and K, hydrocarbons, and sterols.
Functions of lipids
Lipids fulfill a broad and diverse range of functions that extend far beyond their role as energy reserves. Owing to their chemical heterogeneity and to the coexistence, in many lipid molecules, of polar and non-polar regions, lipids are uniquely suited to participate in a wide variety of biological processes.
At the cellular level, lipids contribute to the organization, stability, and dynamic properties of membranes, influencing their permeability, fluidity, and interactions with proteins. Beyond these structural roles, lipid molecules and their metabolic derivatives are central to intracellular signaling, intercellular communication, and hormonal regulation.
At the level of the whole organism, lipids are involved in energy storage and utilization, protection and insulation of tissues, digestion and absorption of nutrients, and the elimination of excess cholesterol. In addition, certain lipids participate in interactions with the external environment, affecting sensory perception and, in many species, mediating chemical communication.
The main functional roles of lipids are outlined below.
| Functional category | Typical examples |
|---|---|
| Energy storage and supply | Triglycerides stored in adipose tissue; fatty acids used as metabolic fuel |
| Structural components of membranes | Phospholipids, cholesterol, glycolipids |
| Regulatory and signaling molecules | Diacylglycerol, ceramides, sphingosine, platelet-activating factor |
| Hormonal function | Steroid hormones (estrogens, androgens, cortisol); eicosanoids (prostaglandins, leukotrienes, thromboxanes) |
| Protective and insulating roles | Subcutaneous fat; structural fat pads; myelin sheath |
| Digestive and absorptive functions | Bile salts; dietary lipids facilitating gastric emptying and pancreatic secretion |
| Excretory function | Elimination of cholesterol through bile salts and fecal excretion |
| Ecological and sensory roles | Pheromones; lipids contributing to food texture and flavor |
Energy and nutrition
Fatty acids, stored in cells as triglycerides, are one of the major energy sources in humans. They are also the most energy-dense fuel, providing on average 9 kcal/g, whereas carbohydrates and proteins provide about 4 kcal/g.
Some lipids are essential nutrients. These include vitamin A, which is essential for healthy vision; vitamin D, which is essential for calcium metabolism; vitamin E, which prevents the autoxidation of unsaturated lipids; vitamin K, which is essential for normal blood clotting; and the essential fatty acids, namely linoleic acid and α-linolenic acid, which are the precursors of the omega-6 and omega-3 polyunsaturated fatty acid families, respectively.
Structural and membrane functions
Phospholipids, cholesterol, and glycolipids, together with proteins, are the fundamental building blocks of biological membranes. In addition, lipids can act as receptors, antigens, and membrane anchors for proteins in the plasma membrane. By influencing membrane structure, they also modulate the activity and functionality of membrane-associated enzymes.
Regulatory and hormonal functions
Many lipids act as regulators of intracellular processes. Examples include diacylglycerol, ceramides, sphingosine, and platelet-activating factor, all of which participate in intracellular signaling pathways.
Many hormones are lipids. Steroid hormones, such as estrogens, androgens, and cortisol, are synthesized from cholesterol. In contrast, prostaglandins, prostacyclins, leukotrienes, thromboxanes, and other related compounds, collectively known as eicosanoids, are derived from omega-3 and omega-6 polyunsaturated fatty acids, such as arachidonic acid, eicosapentaenoic acid, and docosahexaenoic acid.
Protective and insulating functions
Some fat deposits are not mobilized during fasting and are classified as structural fat. Their function is to hold organs and nerves in the correct position and to protect them against traumatic injuries and shock. Typical examples include the fat pads on the palms of the hands and the soles of the feet, which protect the underlying bones from mechanical pressure.
In humans, a subcutaneous layer of fat is present that insulates the body, reducing heat loss and contributing to the maintenance of body temperature.
In the epidermis, lipids play a key role in maintaining the water barrier of the skin.
Lipids also act as electrical insulators of axons. Axons are repeatedly wrapped by the plasma membranes of Schwann cells in the peripheral nervous system and by oligodendrocytes in the central nervous system. This multilayered coating is known as the myelin sheath.
Digestive and excretory functions
In the digestive tract, lipids facilitate digestion by reducing gastric secretion, slowing gastric emptying, and stimulating biliary and pancreatic secretion.
Bile salts are natural detergents synthesized in the liver and secreted into bile. They solubilize phospholipids and cholesterol in bile and allow the secretion of cholesterol into the intestine. The excretion of cholesterol and bile salts represents the major pathway by which cholesterol is eliminated from the body. Bile salts also play a crucial role in lipid digestion and absorption, as well as in the absorption of fat-soluble vitamins in the intestine.
Ecological and sensory functions
In many animals, certain lipids are secreted into the external environment, where they act as pheromones that attract or repel other organisms. Finally, lipids influence the texture and flavor of food, thereby affecting its palatability.
Digestion
Unlike carbohydrate digestion and the absorption of monosaccharides, as well as protein digestion and the absorption of amino acids, the digestion of lipids and the absorption of their digestion products must take into account the poor solubility, or complete insolubility, of many of the molecules involved in the aqueous environment of the intestine.
Lipid digestion begins in the mouth, continues in the stomach, and is completed in the duodenum, while the products of digestion are primarily absorbed in the subsequent segments of the small intestine (jejunum and ileum).
Once lipids reach the stomach, non-polar lipids aggregate into droplets. The formation of these droplets is favored by the stirring and mixing actions within the stomach and by amphipathic molecules released through the action of lingual and gastric lipases, namely short-chain fatty acids, medium-chain fatty acids, and diacylglycerols. These molecules act as emulsifiers by exposing their hydrophilic groups to the aqueous environment and their hydrophobic groups to the non-polar lipid core, thereby creating a hydrophilic surface that can interact stably with the surrounding aqueous medium.
In the duodenum, lipid droplets are further emulsified by bile salts and phospholipids present in bile, which is secreted by the gallbladder. This process leads to the formation of progressively smaller droplets, thereby increasing the surface area available for the action of pancreatic enzymes responsible for lipid digestion.
Intestinal absorption
With the exception of short- and medium-chain fatty acids, the absorption of lipid digestion products requires the formation of structures that transport non-polar molecules to the luminal surface of enterocytes. These structures are called mixed micelles. They are smaller than lipid droplets and are formed by bile salts and lipid digestion products. Their structure resembles that of membrane bilayer discs excised from a biological membrane, in which bile salts are arranged along the edges of the disc, with their hydrophilic regions facing the external aqueous environment and their hydrophobic regions oriented toward the center of the disc.
Mixed micelles allow lipid concentrations near the luminal surface of enterocytes to increase by up to 1,000-fold, thereby facilitating lipid absorption. The concentration gradient between the intestinal lumen and the interior of the enterocyte is also maintained by the rapid intracellular re-esterification of absorbed lipids into cholesterol esters, triglycerides, and phospholipids.
The absorption of lipid digestion products occurs through passive diffusion and through facilitated transport mediated by specific protein carriers.
References
- Akoh C.C., Min D.B. Food lipids: chemistry, nutrition, and biotechnology. 4th Edition. Boca Raton: CRC Press, 2017. doi:10.1201/9781315151854
- Chow Ching K. Fatty acids in foods and their health implications. 3rd Edition. CRC Press, 2008.
- Dakal T.C., Xiao F., Bhusal C.K., Sabapathy P.C., Segal R., Chen J., Bai X. Lipids dysregulation in diseases: core concepts, targets and treatment strategies. Lipids Health Dis 2025;24(1):61. doi:10.1186/s12944-024-02425-1
- Guyton A.C., Hall J.E. Textbook of medical physiology. 14th Edition. Philadelphia: Elsevier, 2021.
- Heilman D., Woski S., Voet D., Voet J.G., Pratt C.W. Fundamentals of biochemistry: life at the molecular level. 6th Edition. Wiley, 2023.
- Rosenthal M.D., Glew R.H. Medical biochemistry – Human metabolism in health and disease. John Wiley & Sons, 2009.
- Stipanuk M.H., Caudill M.A. Biochemical, physiological, and molecular aspects of human nutrition. 4th Edition. St. Louis: Elsevier, 2018.