In 1956, Robert Sidney Cahn, Christopher Ingold, and Vladimir Prelog developed a nomenclature system which, based on a few simple rules, allows the assignment of the absolute configuration of each chiral center in a molecule.[1][2]
This nomenclature system, called the RS system or the Cahn–Ingold–Prelog (CIP) system, when combined with the IUPAC system of nomenclature, makes it possible to name chiral molecules accurately and unambiguously, even when more than one asymmetric center is present.[3]
In most cases, chiral molecules are able to rotate plane-polarized light when it passes through a solution containing them. In this regard, it should be emphasized that the sign of the rotation of plane-polarized light caused by a chiral compound provides no information about the RS configuration of its chiral centers.
The Fischer–Rosanoff convention is another way to describe the configuration of chiral molecules.[4] However, compared with the RS system, it labels the molecule as a whole rather than each individual chiral center and is often ambiguous for molecules with two or more chiral centers.[5]
Summary: Key Points
-
- Definition: a nomenclature system used to assign the absolute configuration of chiral centers in a molecule.
- Priority Rules: a set of three rules used to determine the R or S configuration based on the atomic number of bonded atoms, the sequence of successive atoms, and the presence of multiple bonds.
- Biochemical Context: allows the unambiguous classification of molecules with one or more chiral centers, such as amino acids and monosaccharides.
- Optical Activity Independence: the RS configuration is a structural descriptor and does not predict the direction in which a molecule rotates plane-polarized light (+/−).
Contents
Priority rules of the RS system
The RS system assigns a priority sequence to the groups attached to a chiral center. By tracing a curved arrow from the highest-priority group to the lowest-priority group (excluding the group positioned away from the observer), each chiral center is labeled as either R or S.[6][7]
First rule
A priority sequence is assigned to the groups based on the atomic number of the atoms directly bonded to the chiral center.
The atom with the highest atomic number is given the highest priority, while the atom with the lowest atomic number is given the lowest priority.
For example, if an oxygen atom (O, atomic number 8), a carbon atom (C, atomic number 6), a chlorine atom (Cl, atomic number 17), and a bromine atom (Br, atomic number 35) are attached to the chiral center, the priority order is:
Br > Cl > O > C
For isotopes, the atom with the higher atomic mass is assigned the higher priority.[8]
Second rule
When different groups are attached to a chiral center through identical atoms, the priority sequence is determined by examining the atomic numbers of the next set of atoms bonded, moving outward from the chiral center until the first point of difference is found.
For example, consider the following groups attached to a chiral center: –CH3, –CH2CH3 and –CH2OH. All of these have a carbon atom directly bonded to the chiral center. To assign priority, the atoms bonded to each of these carbons are examined:
- methyl group (–CH3): H, H, H
- ethyl group (–CH2CH3): H, H, C
- hydroxymethyl group (–CH2OH): H, H, O
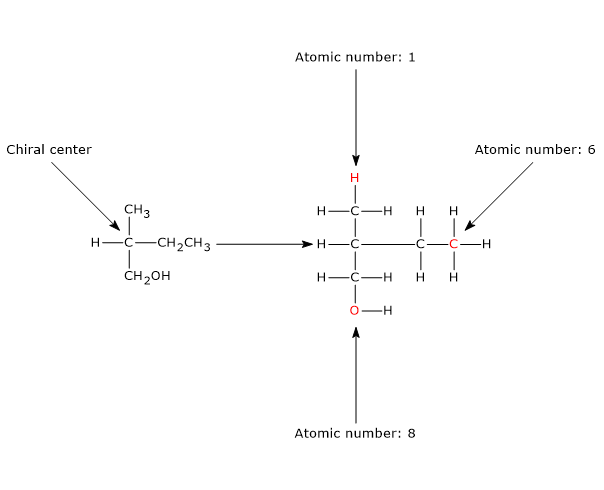
Since oxygen has a higher atomic number than carbon, and carbon has a higher atomic number than hydrogen, the order of priority is:
–CH2OH > –CH2CH3 > –CH3
A typical priority order for common substituents is:
–I > –Br > –Cl > –SH > –OR > –OH > –NHR > –NH2 > –COOR > –COOH > –CHO > –CH2OH > –C6H5 > –CH3 > –2H > –1H
Note: 2H and 1H refer to the isotopes deuterium and protium, respectively.
It is important to note that if two or more groups attached to a chiral center have identical priority, the center is not chiral.
Determining clockwise and counterclockwise orientation
Once the priority sequence has been established, the molecule is oriented so that the group with the lowest priority points away from the observer, behind the chiral center. A circular arrow is then traced from the highest-priority group to the next in sequence.
- If the arrow moves in a clockwise direction, the configuration is R (from the Latin rectus, meaning “right”).
- If the arrow moves in a counterclockwise direction, the configuration is S (from the Latin sinister, meaning “left”).[8]
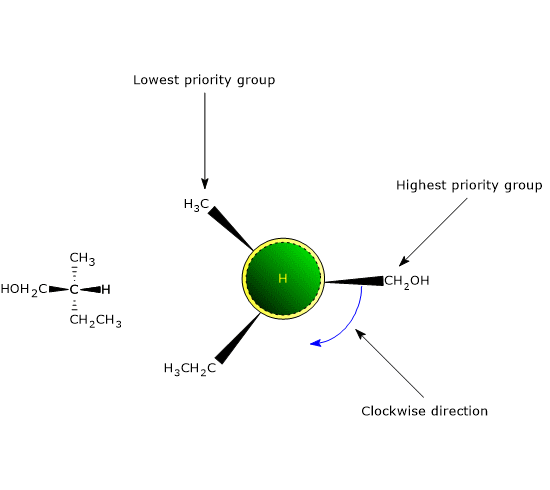
Clockwise Tracing of Substituents at a Chiral Center
Third rule
The third rule of the RS system is used to determine the configuration of a chiral center when one or more of the attached groups contain double or triple bonds.
To assign priorities correctly in such cases, atoms involved in double or triple bonds are treated as if they were duplicated or triplicated, respectively.
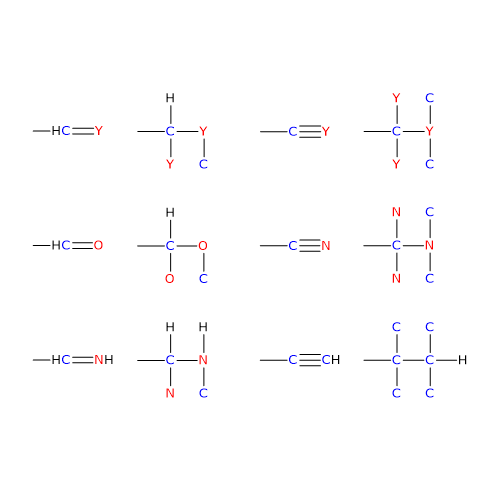
In the case of a C=Y double bond, the carbon is considered to be bonded to two Y atoms, and the Y atom is considered to be bonded to two carbon atoms.
In the case of a C≡Y triple bond, the carbon is treated as being bonded to three Y atoms, and the Y atom as being bonded to three carbon atoms.[8]
RS system and molecules with multiple chiral centers
When two or more chiral centers are present in a molecule, each center is analyzed independently using the rules previously described.
Consider 2,3-butanediol. This molecule contains two chiral centers, carbon 2 and carbon 3, and exists as three stereoisomers: two enantiomers and one meso compound. What is the RS configuration of the chiral centers in the enantiomer shown in the figure?
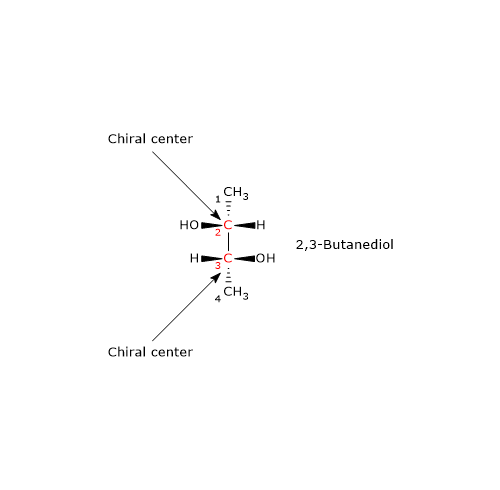
Let us analyze carbon 2. The order of priority of the attached groups is:
–OH > –CH(OH)CH3 > –CH3 > –H
(Here, –CH(OH)CH3 represents the portion of the molecule extending toward carbon 3.)
The molecule is then rotated so that the hydrogen, the group with the lowest priority, points away from the observer (i.e., behind the chiral center). A path is traced from –OH (highest priority) to –CH3. This path moves in a clockwise direction, so the configuration at carbon 2 is R.
Applying the same procedure to carbon 3, its configuration is also found to be R.
Therefore, the enantiomer shown in the figure is (2R,3R)-2,3-butanediol.
Amino acids and glyceraldehyde
In the Fischer–Rosanoff convention, all proteinogenic amino acids are classified as L-amino acids. In the RS system, with the exception of glycine, which is not chiral, and cysteine, which, due to the presence of a thiol group, is classified as (R)-cysteine, all other proteinogenic amino acids are (S)-amino acids.
Threonine and isoleucine each have two chiral centers: the α-carbon and an additional carbon atom in the side chain. Both exist as three stereoisomers: two enantiomers and one meso compound. The naturally occurring forms found in proteins are:
- (2S,3R)-threonine;
- (2S,3S)-isoleucine.
These correspond, in the Fischer–Rosanoff convention, to L-threonine and L-isoleucine, respectively.
In the RS system, L-glyceraldehyde is classified as (S)-glyceraldehyde; accordingly, D-glyceraldehyde is classified as (R)-glyceraldehyde.[9]
References
- ^ Cahn R.S., Ingold C., Prelog V. Specification of molecular chirality. Angew Chem 1966:5(4); 385-415. doi:10.1002/anie.196603851
- ^ Prelog V. and Helmchen G. Basic principles of the CIP‐system and proposals for a revision. Angew Chem 1982:21(8);567-583. doi:10.1002/anie.198205671
- ^ IUPAC. Compendium of chemical terminology, 2nd ed. (the “Gold Book”). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). Online version (2019-) created by S. J. Chalk. ISBN 0-9678550-9-8. doi:10.1351/goldbook
- ^ Rosanoff M.A. On Fischer’s classification of stereo-isomers. J Am Chem Soc 1906:28(1);114-121. doi:10.1021/ja01967a014
- ^ Heilman D., Woski S., Voet D., Voet J.G., Pratt C.W. Fundamentals of biochemistry: life at the molecular level. 6th Edition. Wiley, 2023.
- ^ Garrett R.H., Grisham C.M. Biochemistry. 7th Edition. Cengage Learning, 2023.
- ^ Moran L.A., Horton H.R., Scrimgeour K.G., Perry M.D. Principles of Biochemistry. 5th Edition. Pearson, 2012.
- ^ a b c Solomons T.W.G., Fryhle C.B., Snyder S.A. Solomons’ organic chemistry. 12th Edition. John Wiley & Sons Incorporated, 2017.
- ^ Morris D.G., Drayton C., Hepworth J.D. Stereochemistry. Royal society of chemistry. 2001. doi:10.1039/9781847551948