Hydroxycinnamic acids or hydroxycinnamates are phenolic compounds belonging to non-flavonoid polyphenols.
They are present in all parts of fruits and vegetables although the highest concentrations are found in the outer part of ripe fruits, concentrations that decrease during ripening, while the total amount increases as the size of the fruits increases.
Their dietary intake has been associated with the prevention of:
- cardiovascular diseases;
- cancer;
- type-2 diabetes.
These effects do seem to be due not only to their high antioxidant activity, that depends upon the hydroxylation pattern of the aromatic ring, but also to other mechanisms of action such as, e.g., the reduction of intestinal absorption of glucose or the modulation of secretion of some gut hormones.
Contents
Chemical structure
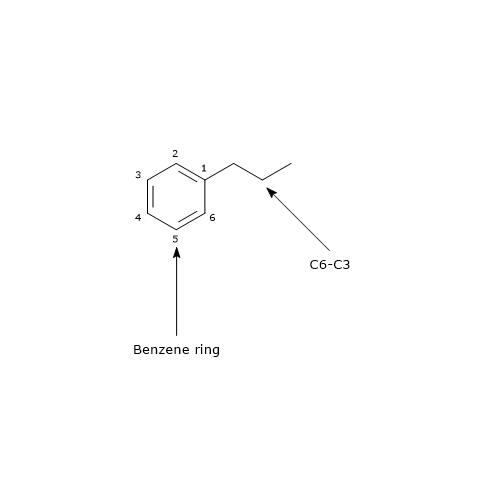
Their basic structure is a benzene ring to which a three carbon chain is attached, structure that is referred to as C6-C3. Therefore they can be included in the phenylpropanoid group.
The main dietary hydroxycinnamates are:
- caffeic acid or 3,4-dihydroxycinnamic acid;
- ferulic acid or 4-hydroxy-3-methoxycinnamic acid;
- sinapic acid or 4-hydroxy-3,5-dimethoxycinnamic acid;
- p-coumaric acid or 4-coumaric acid or 4-hydroxycinnamic acid.
In nature, they are associated with other molecules to form, e.g., glycosylated derivatives or esters of tartaric acid, quinic acid, or shikimic acid. In addition, several hundreds of anthocyanins acylated with the aforementioned hydroxycinnamates have been identified (in descending order with p-coumaric acid, more than 150, caffeic acid, about 100, ferulic acid, about 60, and sinapic acid, about 25).
They are rarely present in the free form, except in processed foods that have undergone fermentation, sterilization or freezing. For example, an overlong storage of blood orange fruits causes a massive hydrolysis of hydroxycinnamic derivatives to free acids, and this in turn could lead to the formation of malodorous compounds such as vinyl phenols, indicators of too advanced senescence of the fruit.
Biosynthesis
Hydroxycinnamate biosynthesis consists of a series of enzymatic reactions subsequent to that catalyzed by phenylalanine ammonia lyase (PAL).
Phenylalanine ⇄ trans-Cinnamic acid + NH3
This enzyme catalyzes the deamination of phenylalanine to yield trans-cinnamic acid, so linking the aromatic amino acid to the hydroxycinnamic acids and their activated forms.
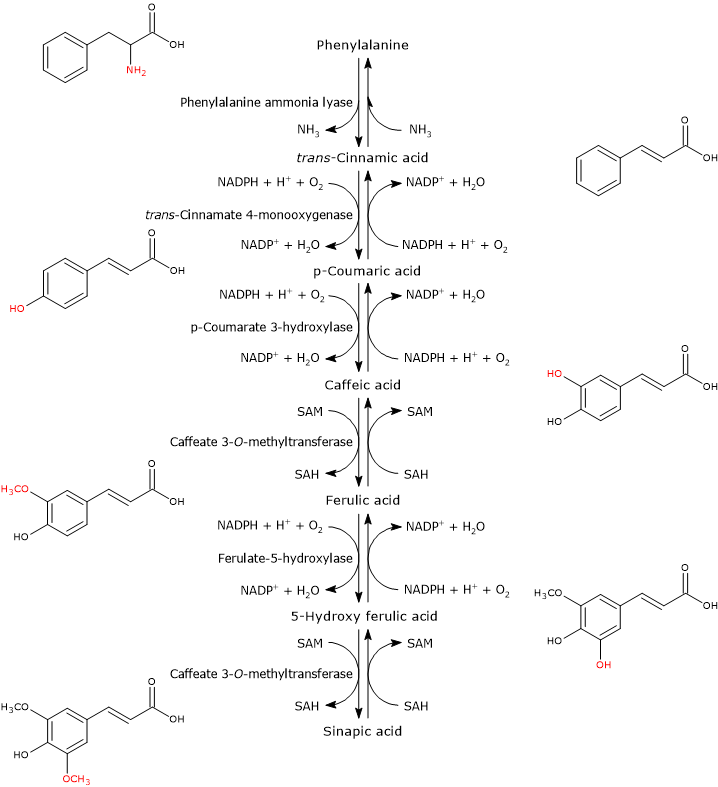
In the first step, a hydroxyl group is attached at the 4-position of the aromatic ring of trans-cinnamic acid to form p-coumaric acid. The reaction catalysed by trans-cinnamate 4-monooxygenase (EC:1.14.14.91).
trans-Cinnamic acid + NADPH + H+ + O2 ⇄ p-Coumaric acid + NADP+ + H2O
The addition of a second hydroxyl group at the 3-position of the ring of p-coumaric acid leads to the formation of caffeic acid. The reaction catalysed by p-coumarate 3-hydroxylase (EC 1.14.13.-).
p-Coumaric acid + NADPH + H+ + O2 ⇄ Caffeic acid + NADP+ + H2O
The O-methylation of the hydroxyl group at the 3-position yields ferulic acid. The reaction catalyzed by caffeate 3-O-methyltransferase (EC:2.1.1.68).
Caffeic acid + SAM ⇄ Ferulic acid + SAH
Ferulic acid is converted into sinapic acid through two reactions: a hydroxylation at the 5-position to form 5-hydroxy ferulic acid, in a reaction catalyzed by ferulate 5-hydroxylase (EC:1.14.-.-), and the subsequent O-methylation of the same hydroxyl group in a reaction catalyzed by caffeate 3-O-methyltransferase.
Ferulic acid + NADPH + H+ + O2 ⇄ 5-Hydroxy ferulic acid + NADP+ + H2O
5-Hydroxy ferulic acid + SA from M ⇄ Sinapic acid + SAH
Hydroxycinnamic acids are not present in high quantities since they are rapidly converted to glucose esters or coenzyme A (CoA) esters, in reactions catalyzed by O-glucosyltransferases and hydroxycinnamate:CoA ligases, respectively. These activated intermediates are branch points, being able to participate in a wide range of reactions such as condensation with malonyl-CoA to form flavonoids, or the NADPH-dependent reduction to form lignans, which are precursors of lignin.
Food sources
Kiwis, blueberries, plums, cherries, apples, pears, chicory, artichokes, carrots, lettuce, eggplant, wheat and coffee are among the richest sources.
Caffeic acid
It is generally, both in the free form and bound to other molecules, the most abundant hydroxycinnamic acid in vegetables and most of the fruits, where it represents between 75 and 100 percent of the hydroxycinnamates.
The richest sources are coffee (drink), carrots, lettuce, potatoes, even sweet ones, and berries such as blueberries, cranberries and blackberries.
Smaller quantities are present in grapes and grape-derived products, orange juice, apples, plums, peaches, and tomatoes.
Caffeic acid and quinic acid bind to form chlorogenic acid, present in many fruit and in high concentration in coffee.
Ferulic acid
It is the most abundant hydroxycinnamic acid in cereals, which are also its main dietary source.
In wheat grain, its content is between 0.8 and 2 g/kg dry weight, which represents up to 90 percent of the total polyphenols. It is found chiefly, up to 98 percent of the total content, in the aleurone layer and pericarp, namely, the outer parts of the grain, and therefore its content in wheat flours depends upon the degree of refining, while the main source is obviously the bran. The molecule is present mainly in the trans form, and esterified with arabinoxylans and hemicelluloses. And in fact, in wheat bran the soluble free form represents only about 10 percent of its total amount. Dimers were also found, which form bridge structures between chains of hemicellulose.
In fruits and vegetables, ferulic acid is much less common than caffeic acid. The main sources are asparagus, eggplant and broccoli; lower quantities are found in blackberries, blueberries, cranberries, apples, carrots, potatoes, beets, coffee and orange juice.
Sinapic acid
The highest amounts are found in citrus peel and seeds (in orange juice, the amount is much lower); appreciable quantities in Chinese cabbage and in some varieties of cranberries.
p-Coumaric acid
High amounts are present in eggplant, the richest source, broccoli and asparagus; other sources are sweet cherries, plums, blueberries, cranberries, citrus peel and seeds, and orange juice.
References
- Andersen Ø.M., Markham K.R. Flavonoids: chemistry, biochemistry, and applications. CRC Press Taylor & Francis Group, 2006
- de la Rosa L.A., Alvarez-Parrilla E., Gonzàlez-Aguilar G.A. Fruit and vegetable phytochemicals: chemistry, nutritional value, and stability. 1th Edition. Wiley J. & Sons, Inc., Publication, 2010
- Manach C., Scalbert A., Morand C., Rémésy C., and Jime´nez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004;79(5):727-747. doi:10.1093/ajcn/79.5.727
- Preedy V.R. Coffee in health and disease prevention. Academic Press, 2014
- Zhao Z., Moghadasian M.H. Bioavailability of hydroxycinnamates: a brief review of in vivo and in vitro studies. Phytochem Rev 2010;9(1):133-145. doi:10.1007/s11101-009-9145-5