Flavonoids are the most abundant polyphenols in human diet, representing about 2/3 of all those ones ingested.
Like other phytochemicals, they are the products of secondary metabolism of plants and, currently, it is not possible to determine precisely their number, even if over 4000 have been identified.
In fruits and vegetables, they are usually found in the form of glycosides and sometimes as acylglycosides, while acylated, methylated and sulfate molecules are less frequent and in lower concentrations.
They are water-soluble and accumulate in cell vacuoles.
Contents
Chemical structure
Their basic structure is a skeleton of diphenylpropane, i.e., two benzene rings linked by a three carbon chain that forms a pyran ring, namely, a heterocyclic ring containing oxygen, closed with the benzene ring A, which is called ring C. Therefore, their structure is also referred to as C6-C3-C6.
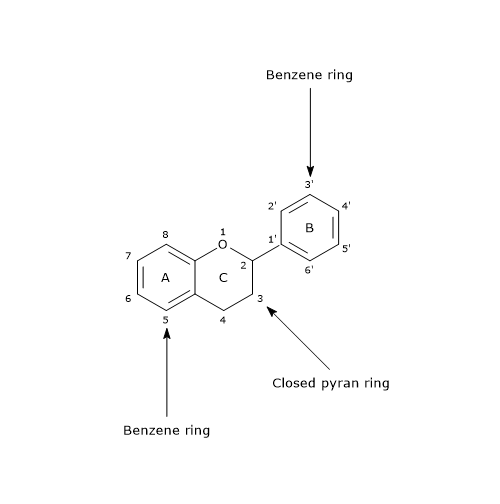
In most cases, B ring is attached to position 2 of C ring, but it can also bind in position 3 or 4. This, together with the structural features of the ring B and the patterns of glycosylation and hydroxylation of the three rings, makes the flavonoids one of the larger and more diversified groups of phytochemicals, so not only of polyphenols, in nature.
Their biological activities, for example they are potent antioxidants, depend both on the structural characteristics and the pattern of glycosylation.
Classification
They can be subdivided into different subclasses depending on the carbon of the C ring on which B ring is attached, and the degree of unsaturation and oxidation of the C ring.
Flavonoids in which B ring is linked in position 3 of the ring C are called isoflavones. Those in which B ring is linked in position 4, neoflavonoids, while those in which the B ring is linked in position 2 can be further subdivided into several subgroups on the basis of the structural features of the C ring. These subgroup are: flavones, flavonols, flavanones, flavanonols, flavanols or catechins and anthocyanins. Finally, flavonoids with open C ring are called chalcones.
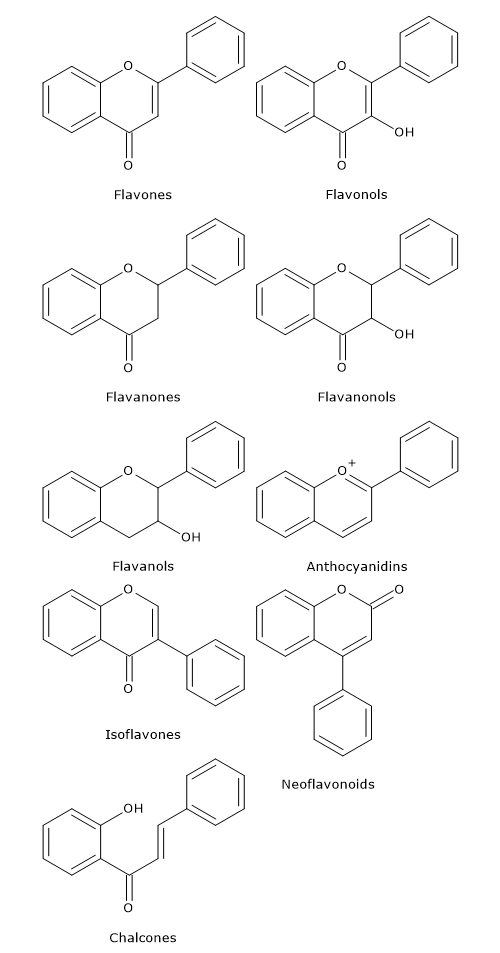
Flavones
They have a double bond between positions 2 and 3 and a ketone in position 4 of the C ring. Most flavones of vegetables and fruits has a hydroxyl group in position 5 of the A ring, while the hydroxylation in other positions, for the most part in position 7 of the A ring or 3′ and 4′ of the B ring may vary according to the taxonomic classification of the particular vegetable or fruit.
Glycosylation occurs primarily on position 5 and 7, methylation and acylation on the hydroxyl groups of the B ring.
Some flavones, such as nobiletin and tangeretin, are polymethoxylated.
Flavonols
Compared to flavones, they have a hydroxyl group in position 3 of the C ring, which may also be glycosylated. Again, like flavones, flavonols are very diverse in methylation and hydroxylation patterns as well, and, considering the different glycosylation patterns, they are perhaps the most common and largest subgroup of flavonoids in fruits and vegetables. For example, quercetin is present in many plant foods.
Flavanones
Flavanones, also called dihydroflavones, have the C ring saturated. Therefore, unlike flavones, the double bond between positions 2 and 3 is saturated and this is the only structural difference between the two subgroups of flavonoids.
The flavanones can be multi-hydroxylated, and several hydroxyl groups can be glycosylated and/or methylated.
Some have unique patterns of substitution, for example, furanoflavanones, prenylated flavanones, pyranoflavanones or benzylated flavanones, giving a great number of substituted derivatives.
Over the past 15 years, the number of flavanones discovered is significantly increased.
Flavanonols
Flavanonols, also called dihydroflavonols, are the 3-hydroxy derivatives of flavanones; they are an highly diversified and multisubstituted subgroup.
Isoflavones
As anticipated, isoflavones are a subgroup of flavonoids in which the B ring is attached to position 3 of the C ring. They have structural similarities to estrogens, such as estradiol, and for this reason they are also called phytoestrogens.
Catechins
Catechins are also referred to flavan-3-ols as the hydroxyl group is almost always bound to position 3 of C ring; they are called flavanols as well.
They have two chirality centers in the molecule, on positions 2 and 3, then four possible diastereoisomers. Epicatechin is the isomer with the cis configuration and catechin is the one with the trans configuration. Each of these configurations has two stereoisomers, namely, (+)-epicatechin and (-)-epicatechin, (+)-catechin and (-)-catechin.
(+)-Catechin and (-)-epicatechin are the two isomers most often present in edible plants.
Another important feature of flavanols, particularly of catechin and epicatechin, is the ability to form polymers, called proanthocyanidins or condensed tannins. The name “proanthocyanidins” is due to the fact that an acid-catalyzed cleavage produces anthocyanidins.
Proanthocyanidins typically contain 2 to 60 monomers of flavanols.
Monomeric and oligomeric flavanols, consisting of 2 to 7 monomers, are strong antioxidants.
Anthocyanidins
Chemically, anthocyanidins are flavylium cations and are generally present as chloride salts.
They are the only colored flavonoids, and, with carotenoids, impart color to plants. Moreover, they are used commercially as food colours.
Anthocyanins are glycosides of anthocyanidins. Sugar units are bound mostly to position 3 of the C ring and they are often conjugated with phenolic acids, such as ferulic acid.
The color of the anthocyanins depends on the pH and also by methylation or acylation at the hydroxyl groups on the A and B rings.
Chalcones
Chalcones and dihydrochalcones are flavonoids with open structure; they are classified as flavonoids because they have similar synthetic pathways.
References
- de la Rosa L.A., Alvarez-Parrilla E., Gonzàlez-Aguilar G.A. Fruit and vegetable phytochemicals: chemistry, nutritional value, and stability. 1th Edition. Wiley J. & Sons, Inc., Publication, 2010
- Han X., Shen T. and Lou H. Dietary polyphenols and their biological significance. Int J Mol Sci 2007;9:950-988. doi:10.3390/i8090950
- Manach C., Scalbert A., Morand C., Rémésy C., and Jime´nez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004;79(5):727-747. doi:10.1093/ajcn/79.5.727
- Panche A.N., Diwan A.D., and Chandra S.R. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. doi:10.1017/jns.2016.41
- Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010;2:1231-1246. doi:10.3390/nu2121231