Olive oil is a liquid fat obtained by pressing whole olives, the fruit of the olive tree (Olea europaea).
It is a staple ingredient in Mediterranean diet and is widely used for cooking, salad dressings, and other culinary purposes. Olive oil is also known for its health benefits, as it is rich in monounsaturated fats and antioxidants.
From a chemical point of view, we can identify in the olive oil two fractions, depending on the behavior in the presence of heating and strong alkaline solutions:
- the saponifiable fraction, which represents 98-99 percent of the total weight, is composed of lipids that form soaps in the above conditions;
- the unsaponifiable fraction, which represents the remaining 1-2 percent of the total weight, is composed of substances that fail to form soaps in the above conditions.
Contents
Saponifiable fraction
It is composed of saturated fatty acids and unsaturated fatty acids, esterified almost entirely to glycerol to form triglycerides or triacylglycerols. To a much lesser extent, diglycerides or diacylglycerols, monoglycerides or monoacylglycerols, and free fatty acids are also found.
Unsaturated fatty acids make up 75 to 85 percent of the total fatty acids. Oleic acid (O) and linoleic acid (L) are the most abundant ones; palmitoleic acid, eptadecenoic acid, gadoleic acid, and alpha-linolenic acid (Ln) are present in lower/trace amounts.
| IOOC requirements for olive oil | ||
| Fatty acids | Number of carbons | Allowable range percent |
| Myristic acid | C14:0 | <0.03 |
| Palmitic acid | C16:0 | 7.5-20 |
| Palmitoleic acid | C16:1 | 0.3-3.5 |
| Heptadecanoic acid | C17:0 | ≤0.3 |
| Heptadecenoic acid | C17:1 | ≤0.3 |
| Stearic acid | C18:0 | 0.5-5.0 |
| Oleic acid | C18:1 | 55.0-83.0 |
| Linoleic acid | C18:2 | 2.5-21.0 |
| Alpha-linolenic acid | C18:3 | ≤1.0 |
| Arachidic acid | C20:0 | ≤0.6 |
| Gadoleic acid | C20:1 | ≤0.4 |
| Behenic acid | C22:0 | ≤0.2 |
| Lignoceric acid | C24:0 | ≤0.2 |
Oleic acid is the major fatty acid in olive oils. According to the rules laid down by the International Olive Oil Council (IOOC), its concentration must range from 55 percent to 83 percent of total fatty acids.
Linoleic acid is the most abundant polyunsaturated fatty acid in olive oil; its concentration must vary between 2.5 percent and 21 percent (IOOC). Because of its high degree of unsaturation, it is subject to oxidation; this means that an oil high in linoleic acid becomes rancid easily, and thus it may be stored for a shorter time.
In mediterranean cuisine, olive oil is the main source of fat: therefore, oleic acid, among monounsaturated fatty acids, and linoleic acid, among polyunsaturated fatty acids, are the most abundant fatty acids.
alpha-Linolenic acid must be present in very low amount, according to the IOOC standards ≤1 percent. It is an omega-3 polyunsaturated fatty acid, which may have health benefits. However, because of to its high degree of unsaturation, higher than that of linoleic acid, it is very susceptible to oxidation, and therefore it promotes rancidity of the olive oil that contains it.
Saturated fatty acids make up 15 to 25 percent of the total fatty acids.
Palmitic acid (P), 5-20 percent, and stearic acid (S), 0.5-5 percent, are the most abundant saturated fatty acids; myristic acid, heptadecanoic acid, arachidic acid, behenic acid and lignoceric acid may be present in trace amounts.
The presence of fatty acids that should be absent or present in amounts different than those found is a marker of adulteration with other vegetable oils. On this regard, particular attention is paid to myristic, arachidic, behenic, lignoceric, gadoleic and alpha-linolenic acids, whose limits are set by IOOC.
Fatty acid composition is influenced by several factors.
- The climate.
- The latitude.
- The zone of production.
Italian, Spanish and Greek olive oils are high in oleic acid and low in palmitic and linoleic acids, while Tunisian olive oils are high in palmitic and linoleic acids but lower in oleic acid. Therefore, oils can be divided into two groups:
one rich in oleic acid and low in palmitic and linoleic acids;
the other high in palmitic and linoleic acids and low in oleic acid.
- The cultivar.
- The degree of olive ripeness at the time of oil extraction.
It should be noted that oleic acid is formed first in the fruit, and data seem to indicate a competitive relationship between oleic acid and palmitic, palmitoleic, and linoleic acids.
Triglycerides
As previously said, fatty acids in olive oil are almost entirely present as triglycerides.
In small percentage, they are also present as diglycerides, monoglycerides, and in free form.
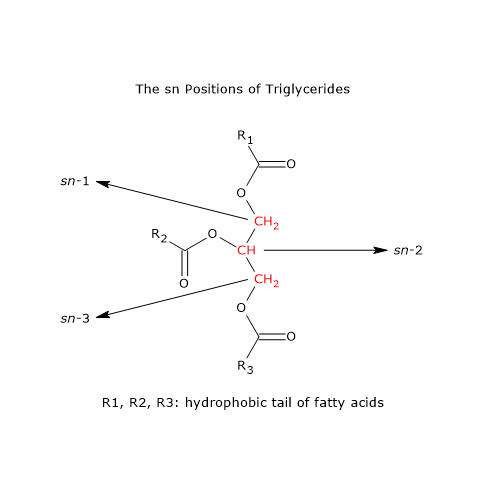
During triglyceride biosynthesis, thanks to the presence of specific enzymes, only about 2 percent of glycerol binds palmitic acid in the sn-2 position. The percentage of stearic acid in the sn-2 position is very low, as well. For the most part, the sn-2 position is occupied by oleic acid.
On the contrary, if we consider oils that have undergone a nonenzymatic esterification, the percentage of palmitic acid in the sn-2 position increases significantly.
Note: sn = Stereospecific numbering
Among triglycerides present in significant proportions in olive oil, there are:
- OOO: 40-59 percent;
- POO: 12-20 percent;
- OOL: 12.5-20 percent;
- POL: 5.5-7 percent;
- SOO: 3- 7 percent.
POP, POS, OLnL, OLnO, PLL, PLnO are present in smaller amounts.
Trilinolein (LLL) is a triglyceride that contains three molecules of linoleic acid. Its low content is an indicator of an oil of good quality.
Triglycerides containing three saturated fatty acids or three molecules of alpha-linolenic acid have not been reported.
Diglycerides and monoglycerides
Their presence is due to an incomplete synthesis and/or a partial hydrolysis of triglycerides.
The content of diglycerides in virgin olive oil ranges from 1 percent to 2.8 percent. 1,2-Diglycerides prevail in fresh olive oil, representing over 80 percent of the diglycerides. During oil storage, isomerization occurs with a progressive increase of the more stable 1-3 isomers, which after about 10 months become the major isomers.
Therefore, the ratio 1,2/1,3-diglycerides may be used as an indicator of the age of the oil.
Monoglycerides are present in amounts lower than diglycerides, <0.25 percent, with 1-monoglycerides far more abundant than 2-monoglycerides.
Unsaponifiable fractions
It is composed of a large number of different molecules, very important from a nutritional point of view, as they contribute significantly to the health effects of olive oil.
Furthermore, they are responsible for the stability and the taste of olive oil, and are also used to detect adulteration with other vegetable oils.
This fraction includes tocopherols, sterols, polyphenols, pigments, hydrocarbons, aromatic and aliphatic alcohol, triterpene acids, waxes, and minor constituents.
Their content is influenced by factors similar to those seen for fatty acid composition, such as:
- the cultivar;
- the degree of ripeness of the olive;
- the zone of production;
- the crop year and olive harvesting practices;
- the storage time of olives;
- the oil extraction process;
- the storage conditions of the oil.
It should be noted that many of these compounds are not present in refined olive oils, as they are removed during the refining processes.
Polyphenols
They make up 18 to 37 percent of the unsaponifiable fraction.
They are a very heterogeneous group of molecules with nutritional and organoleptic properties; for example, oleuropein and hydroxytyrosol give oil its bitter and pungent taste.
For a more extensive discussion, see the article Polyphenols in olive oil.
Hydrocarbons
They make up 30 to 50 percent of the unsaponifiable fraction.
Squalene and beta-carotene are the main molecules.
Squalene, isolated for the first time from shark liver, is the major constituent of the unsaponifiable fraction, and constitutes more than 90 percent of the hydrocarbons. Its concentration ranges from 200 to 7500 mg/kg of olive oil.
It is an intermediate in the biosynthesis of the four-ring structure of steroids, and it seems to be responsible of several health effects of olive oil.
In the hydrocarbon fraction of virgin olive oil, n-paraffins, diterpene and triterpene hydrocarbons, isoprenoidal polyolefins are also found.
Beta-carotene acts both as antioxidant, protecting oil during storage, and as dye.
Sterols
They are important lipids of olive oil, and are:
- linked to many health benefits for consumers;
- important to the quality of the oil;
- widely used for checking its genuineness.
On this regard, it is to underline that sterols are species-specific molecules; for example, the presence of high concentrations of brassicasterol, a sterol typically found in Brassicaceae (Cruciferae) family, such as rapeseed, indicates adulteration of olive oil with canola oil.
Four classes of sterols are present in olive oil: common sterols, 4-methylsterols, triterpene alcohols, and triterpene dialcohols. Their content ranges from 1000 mg/kg, the minimum value required by the IOOC standard, to 2000 mg/kg. The lowest values are found in refined oils because of the refining processes may cause losses up to 25 percent.
Common sterols or 4-alpha-desmethylsterols
Common sterols are present mainly in the free and esterified form; however they have been also found as lipoproteins and sterylglucosides.
The main molecules are beta-sitosterol, which makes up 75 to 90 percent of the total sterol, Δ5-avenasterol, 5 to 20 percent, and campesterol, 4 percent. Other components found in lower amounts or traces are, for example, stigmasterol, 2 percent, cholesterol, brassicasterol, and ergosterol.
4-Methylsterols
They are intermediates in the biosynthesis of sterols, and are present both in the free and esterified form. They are present in small amounts, much lower than those of common sterols and triterpene alcohols, varying between 50 and 360 mg/kg. The main molecules are obtusifoliol, cycloeucalenol, citrostadienol, and gramisterol.
Triterpene alcohols or 4,4-dimethylsterols
They are a complex class of sterols, present both in the free and esterified form. They are found in amounts ranging from 350 to 1500 mg/kg.
The main components are beta-amyrin, 24-methylenecycloartanol, cycloartenol, and butyrospermol; other molecules present in lower/trace amounts are, for example, cyclosadol, cyclobranol, germanicol, and dammaradienol.
Triterpene dialcohols
The main triterpene dialcohols found in olive oil are erythrodiol and uvaol.
Erythrodiol is present both in the free and esterified form; in virgin olive oil, its level varies between 19 and 69 mg/kg, and the free form is generally lower than 50 mg/kg.
Tocopherols
They make up 2 to 3 percent of the unsaponifiable fraction, and include vitamin E.
Of the eight E-vitamers, alpha-tocopherol represents about 90 percent of tocopherols in virgin olive oil. It is present in the free form and in very variable amount, but on average higher than 100 mg/kg of olive oil. Thanks to its in vivo antioxidant properties, its presence is a protective factor for health. Alpha-tocopherol concentration seems to be related to the high levels of chlorophylls and to the concomitant requirement for deactivation of singlet oxygen.
Beta-tocopherol, delta-tocopherol, and gamma-tocopherol are usually present in low amounts.
Pigments
In this group we find chlorophylls and carotenoids.
In olive oil, chlorophylls are present as phaeophytins, mainly phaeophytin a, namely, a chlorophyll from which magnesium has been removed and substituted with two hydrogen ions, and confer the characteristic green color to olive oil. They are photosensitizer molecules that contribute to the photooxidation of olive oil itself.
Beta-carotene and lutein are the main carotenoids in olive oil. Several xanthophylls are also present, such as antheraxanthin, beta-cryptoxanthin, luteoxanthin, mutatoxanthin, neoxanthin, and violaxanthin.
Olive oil’s color is the result of the presence of chlorophylls and carotenoids and of their green and yellow hues. Their presence is closely related.
Triterpene acids
They are important components of the olive, and are present in trace amounts in the oil.
Oleanolic and maslinic acids are the main triterpene acids in virgin olive oil: they are present in the olive husk, from which they are extracted in small amount during processing.
Aliphatic and aromatic alcohols
Fatty alcohols and diterpene alcohols are the most important ones.
Aliphatic alcohols have a number of carbon atoms between 20 and 30, and are located mostly inside the olive stones, from where they are partially extracted by milling.
Fatty alcohols
They are linear saturated alcohols with more than 16 carbon atoms.
They are found in the free and esterified form and are present, in virgin olive oil, in amount not generally higher than 250 mg/kg.
Docosanol (C22), tetracosanol (C24), hexacosanol (C26), and octacosanol (C28) are the main fatty alcohols in olive oil, with tetracosanol and hexacosanol present in larger amounts.
Waxes, which are minor constituents of olive oil, are esters of fatty alcohols with fatty acids, mainly of palmitic acid and oleic acid. They can be used as a criterion to discriminate between different types of oils; for example, they must be present in virgin and extra virgin olive oil at levels <150 mg/kg, according to the IOOC standards.
Diterpene alcohols
Geranylgeraniol and phytol are two acyclic diterpene alcohols, present in the free and esterified form. Among esters present in the wax fraction of extra virgin olive oil, oleate, eicosenoate , eicosanoate, docosanoate, and tetracosanoate have been found, mainly as phytyl derivatives.
Volatile compounds
More than 280 volatile compounds have been identified in olive oil, such as hydrocarbons, the most abundant fraction, alcohols, aldehydes, ketones, esters, acids, ethers and many others. However, only about 70 of them are present at levels higher than the perception threshold beyond which they may contribute to the aroma of virgin olive oil.
Minor components
Phospholipids are found among the minor components of olive oil; the main ones are phosphatidylserine, phosphatidylethanolamine, phosphatidylcholine, phosphatidylinositol.
In the unfiltered oils, trace amounts of proteins may be found.
References
- Gunstone F.D. Vegetable oils in food technology: composition, properties and uses. 2th Edition. Wiley J. & Sons, Inc., Publication, 2011
- Caponio F., Bilancia M.T., Pasqualone A., Sikorska E., Gomes T. Influence of the exposure to light on extra virgin olive oil quality during storage. Eur Food Res Technol 2005;221:92-98. doi:10.1007/s00217-004-1126-8
- Servili M., Sordini B., Esposto S., Urbani S., Veneziani G., Di Maio I., Selvaggini R. and Taticchi A. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants 2014;3:1-23. doi:10.3390/antiox3010001