Protein digestion is the physiological process through which proteins are broken down into amino acids by the action of enzymes known as proteases. These enzymes are synthesized and stored within cells in an inactive forms, known as zymogens, to prevent them from digesting themselves and other cellular proteins. They are released and activated only during digestion.[1]
Cooking and chewing facilitate protein digestion, which occurs in two phases: the first in the stomach and the second and principal phase in the duodenum.[2]
Protein digestion primarily serves to meet the body’s requirement for essential amino acids, which, like essential fatty acids (lipid molecules that cannot be synthesized by the body) must therefore be obtained from the diet.[3]
Summary: Key Points
- Protein digestion: the biochemical process of breaking down dietary and endogenous proteins into their constituent amino acids.
- Gastric phase: initiated in the stomach by the action of hydrochloric acid and pepsin, which denature proteins and begin peptide bond hydrolysis.
- Small intestinal phase: occurs through a cascade of pancreatic proteases (activated from their inactive zymogen forms) and is completed by the action of brush border aminopeptidases and dipeptidases.
- Absorption and fate: the released amino acids, dipeptides, and tripeptides are absorbed by enterocytes. Free amino acids then enter the portal circulation for tissue repair, growth, and metabolic synthesis.
Contents
- Digestive enzymes: classification and origin
- Zymogens, enzyme activation, and protein structure
- Role of cooking and chewing
- Gastric phase of protein digestion
- Small intestinale phase of protein digestion
- Efficiency of proteolytic enzymes
- Importance of protein digestion
- Sources of proteins
- References
Digestive enzymes: classification and origin
Protein digestion occurs through the hydrolysis of the peptide bonds that link individual amino acids within the polypeptide chain. These reactions are catalyzed by proteases.[4]
Digestive proteases, which are specific for amino acid side chains, are hydrolases that can be divided into two main classes:
- endopeptidases, which hydrolyze peptide bonds within the polypeptide chain and are produced by the stomach and the exocrine pancreas;
- exopeptidases, which can be further subdivided into two groups:
- carboxypeptidases, which remove amino acids from the C-terminal end and are produced by the exocrine pancreas;
- aminopeptidases, which act on the N-terminal end and are produced by enterocytes.[1]
| Site of synthesis | Zymogen | Active enzyme |
|---|---|---|
| Stomach | Pepsinogen | Pepsin |
| Pancreas | Chymotrypsinogen | α-Chymotrypsin |
| Pancreas | Trypsinogen | Trypsin |
| Pancreas | Procarboxypeptidase A and B | Carboxypeptidase A and B |
| Pancreas | Proelastase | Elastase |
These enzymes are synthesized and secreted as inactive zymogens.[5]
Zymogens, enzyme activation, and protein structure
Within the cell, zymogens are stored in membrane-bound granules called zymogen granules. When the cell is stimulated by a specific signal, the granule membrane fuses with the plasma membrane, and zymogens are released by exocytosis.[1]
Proteases are synthesized in an inactive form to prevent them from digesting themselves and/or cellular proteins before secretion. In these inactive precursors, the active site of the enzyme is “masked”; only after activation can the enzyme interact with its substrate. Activation results from the cleavage of one or more specific peptide bonds, catalyzed by a specific enzyme, leading to the release of one or more segments of the polypeptide chain. This process allows the enzyme to adopt its active three-dimensional conformation in which the active site is exposed and correctly configured.[4][6]
The accumulation of zymogens within granules also represents an additional protective mechanism, as it isolates them from other cellular components.[5]
Role of cooking and chewing
Most proteins in their native conformation are resistant to proteolytic action. This resistance is due to their secondary and tertiary (native) structure, which masks many peptide bonds from enzymatic attack. These structures are stabilized by covalent bonds, such as disulfide bridges between cysteine residues, as well as by non-covalent forces, including ionic interactions, hydrogen bonds, and van der Waals forces.[5]
For effective protein digestion, peptide bonds must therefore be as accessible as possible to proteases. This is achieved outside the body through cooking, and within the body through the acidic environment of the stomach.[2]
Cooking, when not excessive, facilitates protein digestion.
How does this occur?
Like all molecules, proteins are not motionless but continuously vibrate. As temperature increases, proteins vibrate with greater amplitude, eventually disrupting the non-covalent bonds that help maintain their native structure. As a result, a conformational change occurs, i.e., the protein becomes denatured. This process makes internal peptide bonds more accessible to the action of digestive enzymes.[7]
Chewing and insalivation also homogenize and moisten solid food components, thereby facilitating gastric and small intestinal digestion.[8]
Gastric phase of protein digestion
Protein digestion begins in the stomach, where it represents a preparatory stage for the processes that occur in the duodenum.[9]
The presence of food in the stomach stimulates G cells in the mucosa of the gastric antrum and proximal duodenum to synthesize and release the hormone gastrin into the bloodstream. Gastrin stimulates the parietal cells of the gastric glands, located mainly in the fundus and body of the stomach, to secrete hydrochloric acid (HCl) into the gastric lumen. Parietal cells also produce intrinsic factor, a protein that binds vitamin B12, preventing its degradation and enabling its absorption.
The gastric glands also contain:
- mucous neck cells, which produce mucus;
- chief cells, which secrete pepsinogen.
All these substances, together with others such as potassium ions and gastric lipase, constitute the gastric juice, which has a pH ranging from 1 to 2.5.[10]
Because of its low pH, gastric juice exerts an antiseptic effect, killing most bacteria and other foreign cells, and a denaturing effect, as it disrupts the non-covalent bonds that stabilize the native structure of proteins. This denaturation facilitates the access of intestinal proteases to peptide bonds, in a manner similar to the effect of heat during cooking.[11]
Some proteins rich in disulfide bonds, such as keratins, are resistant to acid-induced denaturation and are therefore difficult to digest. In contrast, most globular proteins are hydrolyzed into large peptide fragments (or polypeptides).[12]
Finally, the acidic pH of gastric juice converts pepsinogen, a zymogen, into pepsin, the first enzyme involved in protein digestion.[13]
Pepsin
There are several isoenzymes of pepsinogen, such as type I, synthesized by chief cells in the body and fundus of the stomach, and type II, which is produced throughout the entire organ. All isoenzymes are converted into the active enzyme.[13]
Activation occurs via autocatalysis at pH values below 5 through an intramolecular process that involves the hydrolysis of a specific peptide bond and the release of a small peptide from the N-terminal end of the zymogen. This peptide remains bound to the enzyme and continues to act as an inhibitor until the pH falls below 2 or until it is further degraded by pepsin itself. Consequently, once some pepsin has been formed, it rapidly activates additional pepsinogen molecules.[3]
Pepsin is an endopeptidase with an optimal activity at pH 1.6 and hydrolyzes approximately 10–20% of the proteins present in a meal. Like many digestive enzymes, pepsin exhibits broad substrate specificity and catalyzes the cleavage of peptide bonds adjacent to amino acid residues such as leucine and the aromatic amino acids phenylalanine, tyrosine, and tryptophan. As a result, a mixture of relatively large peptides and a small amount of free amino acids is produced.[1]
The importance of pepsin lies not so much in its direct contribution to protein digestion, which is modest, but in the generation of peptides and amino acids that, at the duodenal level, stimulate the secretion of cholecystokinin (CCK) and thereby promote the duodenal–pancreatic phase of protein digestion.[9]
It should also be noted that pepsin-mediated digestion of collagen, a family of proteins that surround and hold muscle cells together, facilitates access of pancreatic proteases to the intracellular dietary proteins of meat.[14]
Small intestinal phase of protein digestion
When gastric contents enter the duodenum, their acidity stimulates S cells located in the duodenal mucosa and in the proximal part of the jejunum, the next segment of the small intestine, to synthesize and release the hormone secretin into the bloodstream.[9]
Secretin induces the secretion of an alkaline pancreatic juice rich in bicarbonate ions but relatively poor in enzymes. This juice reaches the duodenum via the pancreatic duct, where it neutralizes gastric hydrochloric acid, raising the pH between 6 and 7. Secretin also stimulates bile secretion and inhibits gastrin release.[15]
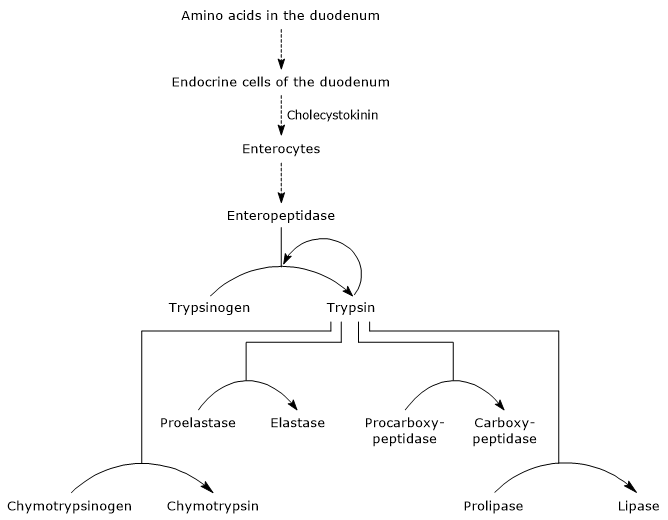
The presence of amino acids in the duodenum stimulates endocrine cells in the duodenum and jejunum to release cholecystokinin into the bloodstream.[5] This hormone, among other functions, stimulates the exocrine pancreas to secrete an enzyme-rich juice in which enzymes are present in the form of zymogens, including:
- trypsinogen, chymotrypsinogen, and proelastase, endopeptidases with substrate specificities different from those of pepsin and from each other;
- procarboxypeptidase A and B, exopeptidases that remove amino acids from the C-terminal end of peptides.[3]
It should be noted that pancreatic proteases, unlike pepsin, exhibit optimal activity at pH values between 7 and 8, corresponding to a neutral or mildly alkaline environment. Thus, the duodenum provides a neutral environment suitable for continuing protein digestion.[1]
Because these proteases have different substrate specificities, peptides generated by one enzyme can serve as substrates for others. Consequently, partially digested proteins entering the duodenum are efficiently hydrolyzed into free amino acids and peptides of 2–8 amino acid residues.[12]
While not directly involved in protein breakdown, pancreatic juice also contains α-amylase, lipase, and nuclease.[3]
Activation of pancreatic zymogens
The first and key step in pancreatic zymogen activation is the conversion of trypsinogen into trypsin by enteropeptidase, also known as enterokinase. This endopeptidase is expressed by duodenal enterocytes and functionally coordinated with CCK-mediated pancreatic secretion.[9] Enteropeptidase catalyzes the cleavage of a specific peptide bond between lysine-6 and isoleucine-7 (in the human sequence) in trypsinogen, resulting in the release of a hexapeptide. This cleavage induces a conformational rearrangement of the protein that leads to its activation, i.e., the formation of trypsin.[12]
Trypsin activates other pancreatic zymogens, including chymotrypsinogen, proelastase, and procarboxypeptidase A and B, as well as additional trypsinogen molecules through autocatalysis. It also cleaves peptide bonds adjacent to lysine and arginine residues in dietary proteins.[16]
As pancreatic zymogens become activated, the protein-digesting capacity of the duodenum progressively increases, a process initiated by a relatively small amount of enteropeptidase.[1]
Pancreatic secretory trypsin inhibitor
An additional protective mechanism against intrapancreatic trypsin activity is the synthesis of an inhibitor known as pancreatic secretory trypsin inhibitor (PSTI).[17]
This molecule, present in pancreatic zymogen granules, binds tightly to the active site of trypsin, thereby inactivating it. In this way, any trypsin generated by premature activation of trypsinogen is blocked, preventing a cascade in which a few activated molecules would otherwise activate all pancreatic zymogens.[12]
In plants, many molecules with similar inhibitory activity exist. An example is the Kunitz trypsin inhibitor, a protein mainly found in soybeans, which forms a highly stable complex with the active site of trypsin.[18][19]
Activation of chymotrypsinogen
The conversion of chymotrypsinogen into chymotrypsin occurs through multiple steps involving both trypsin and activated chymotrypsin itself. In the initial step, trypsin cleaves a specific peptide bond (Arg-15-Ile-16), converting chymotrypsinogen into π-chymotrypsin, which is enzymatically active. Subsequently, through autocatalysis, π-chymotrypsin releases two dipeptides to form δ-chymotrypsin, a more stable intermediate. δ-Chymotrypsin then undergoes two conformational rearrangements: the first yields κ-chymotrypsin, and the second produces α-chymotrypsin, the final active form of the enzyme.
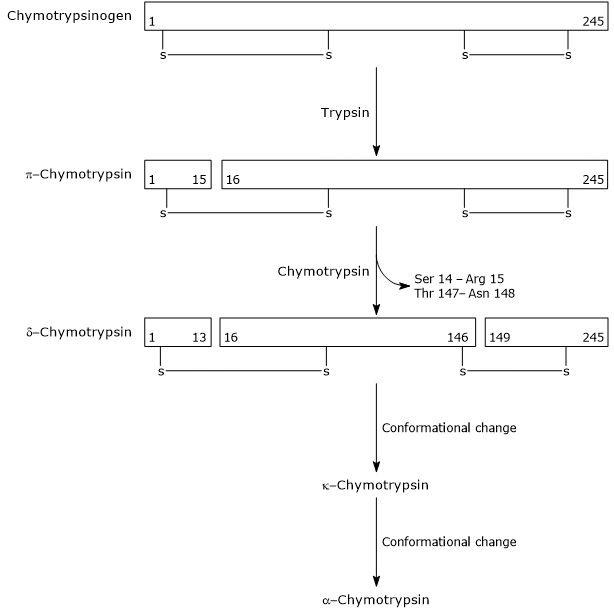
Chymotrypsin hydrolyzes peptide bonds adjacent to phenylalanine, tryptophan, methionine, tyrosine, and leucine residues.[12]
Activation of proelastase
Proelastase is converted into elastase through the removal of a small peptide from its N-terminal end. Elastase, which is less substrate-specific than other digestive proteases, cleaves peptide bonds adjacent to amino acids such as glycine, alanine, and serine.[4]
Activation of procarboxypeptidase
Procarboxypeptidase A is activated to carboxypeptidase A, which removes amino acids with branched or aromatic side chains, such as phenylalanine and valine, from the C-terminal end of peptides.
Procarboxypeptidase B is activated to carboxypeptidase B, which is specific for amino acids with basic side chains, such as lysine and arginine.[5]
Brush border peptidases
The peptides of 2–8 amino acid residues resulting from the activity of activated zymogens are then substrates for aminopeptidases located on the brush border of enterocytes; dipeptidases are also present.[20]
The released amino acids, together with di- and tripeptides, are absorbed by enterocytes. Di- and tri-peptides are hydrolyzed into their constituent amino acids within enterocytes by cytosolic peptidases, which explains why the portal circulation contains almost exclusively free amino acids.[9]
It should be noted that the proteases themselves are ultimately degraded, thereby terminating the digestive process.[3]
Efficiency of proteolytic enzymes
The efficiency of intestinal proteolytic enzymes is evident from the following comparison. In vitro, complete hydrolysis of a protein into its constituent amino acids requires the use of a strong acid (6M HCl) and prolonged heating of the sample at 105 °C. In the gastrointestinal tract, the same result is achieved within a few hours, first in the acidic environment of the stomach and then under the mildly alkaline conditions of the duodenum, at 37 °C.[21]
Importance of protein digestion
The body requires proteins, or more precisely the amino acids that compose them, primarily because essential amino acids cannot be synthesized endogenously and must therefore be obtained from the diet.[5]
Released amino acids are essential for the maintenance, growth, and repair of body tissues, while their contribution as an energy source is, under most conditions, much lower than that of carbohydrates and fatty acids.[9]
The recommended daily protein intake for a healthy adult is approximately 0.9 g/kg of body weight; thus, for a 70-kg individual, this corresponds to about 63 g per day. In adults over 60 years of age, a higher intake is generally recommended to counteract age-related loss of muscle mass, with suggested values of at least 1.0 g/kg of body weight.[22]
Protein digestion is important not only for the host, but also for the gut microbiota. Daily nitrogen loss through feces is approximately 1.6 g, corresponding to about 10 g of protein. Most of this nitrogen is utilized by colonic bacteria for growth and is therefore recovered in the faeces as part of the bacterial biomass.[23]
Sources of proteins
Protein digestion does not involve dietary proteins alone but also includes endogenous proteins. Each day, an additional 50–100 g of endogenous proteins enter the lumen of the gastrointestinal tract as a result of digestive secretions and cellular turnover. These proteins derive from:
- saliva;
- gastric juice;
- pancreatic enzymes and other secretions;
- desquamated intestinal epithelial cells;
- proteins (such as albumin) entering the intestinal lumen from the bloodstream.[1]
References
- ^ a b c d e f g Stipanuk M.H., Caudill M.A. Biochemical, physiological, and molecular aspects of human nutrition. 4th Edition. St. Louis: Elsevier, 2018.
- ^ a b Sensoy I. A review on the food digestion in the digestive tract and the used in vitro models. Curr Res Food Sci 2021;4:308-319. doi:10.1016/j.crfs.2021.04.004
- ^ a b c d e Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 8th Edition. W.H. Freeman and Company, 2021.
- ^ a b c Garrett R.H., Grisham C.M. Biochemistry. 7th Edition. Cengage Learning, 2023.
- ^ a b c d e Heilman D., Woski S., Voet D., Voet J.G., Pratt C.W. Fundamentals of biochemistry: life at the molecular level. 6th Edition. Wiley, 2023.
- ^ Gaisano H.Y., Gorelick F.S. New insights into the mechanisms of pancreatitis. Gastroenterology 2009;136(7):2040-4. doi:10.1053/j.gastro.2009.04.023
- ^ Alberts B., Johnson A., Lewis J., Morgan D., Raff M., Roberts K., Walter P. Molecular Biology of the Cell. 7th Edition. Garland Science, Taylor & Francis Group, 2022.
- ^ Mosca A.C., Chen J. Food oral management: physiology and objective assessment. Curr Opin Food Sci 2016;9:11-20. doi:10.1016/j.cofs.2016.03.003
- ^ a b c d e f Guyton A.C., Hall J.E. Textbook of Medical Physiology. 14th Edition. Philadelphia: Elsevier, 2021.
- ^ Prosapio J.G., Sankar P., Jialal I. Physiology, Gastrin. [Updated 2023 Apr 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/sites/books/NBK534822/
- ^ Martinsen T.C., Fossmark R., Waldum H.L. The phylogeny and biological function of gastric juice-microbiological consequences of removing gastric acid. Int J Mol Sci 2019;20(23):6031. doi:10.3390/ijms20236031
- ^ a b c d e Berg J.M., Tymoczko J.L., Gregory J.G. Jr, Stryer L. Biochemistry. 9th Edition. W.H. Freeman and Company, 2019.
- ^ a b Heda R., Toro F., Tombazzi C.R. Physiology, Pepsin. [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537005/
- ^ Yang M., Yang Z., Everett D. W., Gilbert E. P., Singh H., Ye A. Digestion of food proteins: the role of pepsin. Crit Rev Food Sci Nutr 2025;65(30):6919-6940. doi:10.1080/10408398.2025.2453096
- ^ Afroze S., Meng F., Jensen K., et al. The physiological roles of secretin and its receptor. Ann Transl Med 2013;1(3):29. doi:10.3978/j.issn.2305-5839.2012.12.01
- ^ Freiburghaus A.U., Roduner J., Hadorn H.B. Activation of human pancreatic proteolytic enzymes: the role of enteropeptidase and trypsin. JPGN Rep 2021;2(4):e138. doi:10.1097/PG9.0000000000000138
- ^ Wang G.P., Xu C.S. Pancreatic secretory trypsin inhibitor: More than a trypsin inhibitor. World J Gastrointest Pathophysiol 2010;1(2):85-90. doi:10.4291/wjgp.v1.i2.85
- ^ Li J., Brader G., Palva E.T. Kunitz trypsin inhibitor: an antagonist of cell death triggered by phytopathogens and fumonisin b1 in Arabidopsis. Mol Plant 2008;1(3):482-95. doi:10.1093/mp/ssn013
- ^ Deetanya P., Limsardsanakij K., Sabat G., Pattaradilokrat S., Chaisuekul C., Wangkanont K. Kunitz-type trypsin inhibitor from durian (Durio zibethinus) employs a distinct loop for trypsin inhibition. Protein Sci 2024;33(12):e5230. doi:10.1002/pro.5230
- ^ Anandharamakrishnan C., Moses J.A., Priyanka S. Small intestine, digestion, and nutrient absorption. In: Food digestion and absorption: its role in food product development. Food chemistry, function and analysis. Vol. 42. Cambridge: Royal Society of Chemistry; 2023. doi:10.1039/BK9781839162428-00106
- ^ Santos-Sánchez G., Miralles B., Brodkorb A., Dupont D., Egger L., Recio I. Current advances for in vitro protein digestibility. Front Nutr 2024;11:1404538. doi:10.3389/fnut.2024.1404538
- ^ Nunes E.A., Colenso-Semple L., McKellar S.R., et al. Systematic review and meta-analysis of protein intake to support muscle mass and function in healthy adults. J Cachexia Sarcopenia Muscle 2022;13(2):795-810. doi:10.1002/jcsm.12922
- ^ Bartlett A., Kleiner M. Dietary protein and the intestinal microbiota: an understudied relationship. iScience 2022;25(11):105313. doi:10.1016/j.isci.2022.105313