Olive oil, which is obtained from the pressing of the olives, the fruits of olive tree (Olea europaea), is the main source of lipids in the mediterranean diet, and a good source of polyphenols.
Polyphenols, natural antioxidants, are present in olive pulp and, following pressing, they pass into the oil.
Note: olives are also known as drupes or stone fruits.
The concentration of polyphenols in olive oil is the result of a complex interaction between various factors, both environmental and linked to the extraction process of the oil itself, such as:
- the place of cultivation;
- the cultivars (variety);
- the level of ripeness of the olives at the time of harvesting.
Their level usually decreases with over-ripening of the olives, although there are exceptions to this rule. For example, in warmer climates, olives produce oils richer in polyphenols, in spite of their faster maturation. - the climate;
- the extraction process. In this regard, it is to underscore that the content of polyphenol in refined olive oil is not significant.
Any variation of the concentration of different polyphenols influence the taste, nutritional properties and stability of olive oil. For example, hydroxytyrosol and oleuropein (see below) give extra virgin olive oil a pungent and bitter taste.
Contents
Key polyphenols in olive oil
Among polyphenols in olive oil, there are molecules with simple structure, such as phenolic acids and alcohols, and molecules with complex structure, such as flavonoids, secoiridoids, and lignans.
Flavonoids
Flavonoids include glycosides of flavonols (rutin, also known as quercetin-3-rutinoside), flavones (luteolin-7-glucoside), and anthocyanins (glycosides of delphinidin).
Phenolic acids and phenolic alcohols
Among phenolic acids, the first polyphenols with simple structure observed in olive oil, they are found:
- hydroxybenzoic acids, such as, gallic, protocatechuic, and 4-hydroxybenzoic acids (all with C6-C1 structure).
- hydroxycinnamic acids, such as caffeic, vanillin, syringic, p-coumaric, and o-coumaric acids (all with C6-C3 structure).
Among phenolic alcohols, the most abundant are hydroxytyrosol, also known as 3,4-dihydroxyphenyl-ethanol, and tyrosol, also known as 2-(4-hydroxyphenyl)-ethanol.
Hydroxytyrosol
It can be present as:
- simple phenol;
- phenol esterified with elenolic acid, forming oleuropein and its aglycone;
- part of the molecule verbascoside.
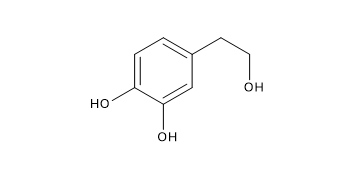
It can also be present in different glycosidic forms, depending on the –OH group to which the glucoside, i.e. elenolic acid plus glucose, is bound.
It is one of the main polyphenols in olive oil, extra virgin olive oil, and olive vegetable water.
In nature, its concentration, such as that of tyrosol, increases during fruit ripening, in parallel with the hydrolysis of compounds with higher molecular weight, while the total content of phenolic molecules and alpha-tocopherol decreases. Therefore, it can be considered as an indicator of the degree of ripeness of the olives.
In fresh extra virgin olive oil, hydroxytyrosol is mostly present in esterified form, while in time, due to hydrolysis reactions, the non-esterified form becomes the predominant one.
Finally, the concentration of hydroxytyrosol is correlated with the stability of olive oil.
Secoiridoids
They are the polyphenols in olive oil with the more complex structure, and are produced from the secondary metabolism of terpenes.
They are glycosylated compounds and are characterized by the presence of elenolic acid in their structure (both in its aglyconic or glucosidic form). Elenolic acid is the molecule common to glycosidic secoiridoids of Oleaceae.
Unlike tocopherols, flavonoids, phenolic acids, and phenolic alcohols, that are found in many fruits and vegetables belonging to different botanical families, secoiridoids are present only in plants of the Oleaceae family.
Ligstroside, oleuropein, demethyloleuropein, and nuzenide are the main secoiridoids.
In particular, oleuropein and demethyloleuropein (as verbascoside) are abundant in the pulp, but they are also found in other parts of the fruit. Nuzenide is only present in the seeds.
Oleuropein
Oleuropein, the ester of hydroxytyrosol and elenolic acid, is the most important secoiridoid, and the main olive oil polyphenol.
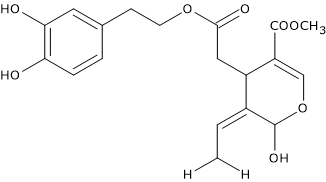
It is present in very high quantities in olive leaves, as also in all the constituent parts of the olive, including peel, pulp and kernel.
Oleuropein accumulates in olives during the growth phase, up to 14% of the net weight; when the fruit turns greener, its quantity reduces. Finally, when the olives turns dark brown, color due to the presence of anthocyanins, the reduction in its concentration becomes more evident.
It was also shown that its content is greater in green cultivars than in black ones.
During the reduction of oleuropein levels (and of the levels of other secoiridoids), an increase of compounds such as flavonoids, verbascosides, and simple phenols can be observed.
The reduction of its content is also accompanied by an increase in its secondary glycosylated products, that reach the highest values in black olives.
Lignans
Lignans, in particular (+)-1- acetoxypinoresinol and (+)-pinoresinol, are another group of polyphenols in olive oil.
(+)-pinoresinol is a common molecule in the lignin fraction of many plants, such as sesame (Sesamun indicum) and the seeds of the species Forsythia, belonging to the family Oleaceae. It has been also found in the olive kernel.
(+)-1- acetoxypinoresinol and (+)-1-hydroxypinoresinol, and their glycosides, have been found in the bark of the olive tree.
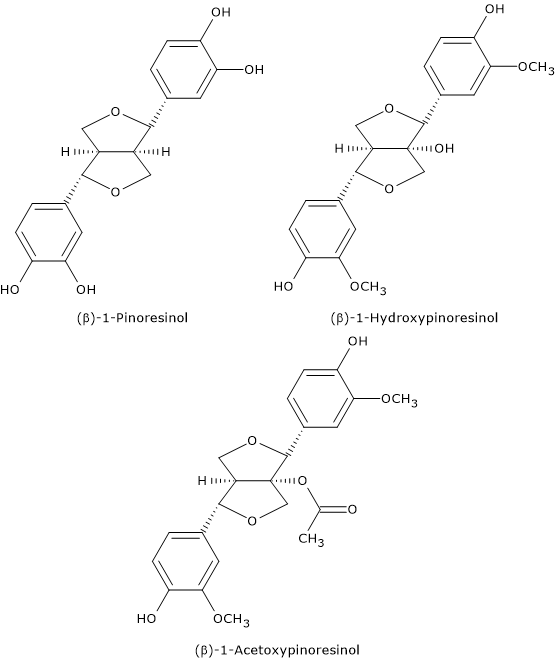
Lignans are not present in the pericarp of the olives, nor in leaves and sprigs that may accidentally be pressed with the olives.
Therefore, how they can pass into the olive oil becoming one of the main phenolic fractions is not yet known.
(+)-1- acetoxypinoresinol and (+)-pinoresinol are absent in seed oils, are virtually absent from refined virgin olive oil, while they may reach a concentration of 100 mg/kg in extra-virgin olive oil.
As seen for simple phenols and secoiridoids, there is considerable variation in their concentration among olive oils of various origin, variability probably related to differences between olive varieties, production areas, climate, and oil production techniques.
References
- Cicerale S., Lucas L. and Keast R. Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010;11: 458-479. doi:10.3390/ijms11020458
- de la Rosa L.A., Alvarez-Parrilla E., Gonzàlez-Aguilar G.A. Fruit and vegetable phytochemicals: chemistry, nutritional value, and stability. 1th Edition. Wiley J. & Sons, Inc., Publication, 2010
- Manach C., Scalbert A., Morand C., Rémésy C., and Jime´nez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004;79(5):727-747. doi:10.1093/ajcn/79.5.727
- Owen R.W., Mier W., Giacosa A., Hull W.E., Spiegelhalder B. and Bartsch H. Identification of lignans as major components in the phenolic fraction. Clin Chem 2000;46:976-988.
- Tripoli E., Giammanco M., Tabacchi G., Di Majo D., Giammanco S. and La Guardia M. The phenolic compounds of olive oil: structure, biological activity and beneficial effects on human health. Nutr Res Rev 2005:18;98-112. doi:10.1079/NRR200495