Carotenoids are a class of fat-soluble pigments widely distributed in nature. They are yellow, orange, and red organic compounds composed of eight isoprene units and characterized by many conjugated double bonds. Their hydrocarbon chain can undergo several modifications that significantly influence their biological properties.[1]
Discovered in the first half of the 1800s, carotenoids are synthesized by all photosynthetic organisms, including plants, macroalgae, and microalgae, as well as by some non-photosynthetic organisms such as certain fungi, bacteria, and insects, for example pea aphids, some species of gall midges, and spider mites. In plants and microalgae, they are synthesized and accumulated in plastids.[2][3]
Together with polyphenols and glucosinolates, they form one of the major groups of phytochemicals.[4]
Over the course of evolution, thanks to their chemical and physical properties, carotenoids have proved extremely versatile, as they perform many functions in both plants and animals, among which their antioxidant activity is particularly important. In humans, some carotenoids also serve as vitamin A precursors.[5][6]
More than 750 different carotenoids have been identified. Over 100 have been found in fruits and vegetables, and about 40 are consumed in significant amounts by humans.[7][8]
Because of their colour, carotenoids are used as food additives.[9][10]
Contents
- History
- Chemical structure
- Isomerism
- Classification
- Solubility
- Colour
- Role in plants
- Food sources
- Food additives
- Carotenoids and human health
- References
History
Carotenoids were discovered in the first half of the nineteenth century.
The first carotenoid to be isolated was β-carotene, in 1831, thanks to the work of the German pharmacist Heinrich Wilhelm Ferdinand Wackenroder, who named “carotene” the yellow pigment crystallized from carrot roots.[2]
Jöns Jacob Berzelius, considered one of the fathers of modern chemistry, named the yellow pigments extracted from autumn leaves “xanthophylls”, from the Greek xanthos (yellow) and phyllon (leaf).[11][12]
Mikhail Semyonovich Tsvet, a Russian botanist who invented chromatography in 1906, succeeded in separating leaf pigments, chlorophylls, carotenes, and xanthophylls, using column chromatography, and called the yellow-to-orange pigments carotenoids.[1]
Chemical structure
Carotenoids are a class of lipids characterized by a backbone composed of eight isoprene units arranged linearly. Therefore, they are terpenes, specifically tetraterpenes, and consist of 40 carbon atoms.[8]
The isoprene units are joined in a 1,5 positional relationship (i.e., head to tail), except in the middle of the hydrocarbon chain, where the relationship is 1,6 (i.e., tail to tail). This inversion results in a symmetrical molecule.[5]
Carotenoids are characterized by the presence of an extended double-bond system, 3 to 13 of which may be conjugated.[13]
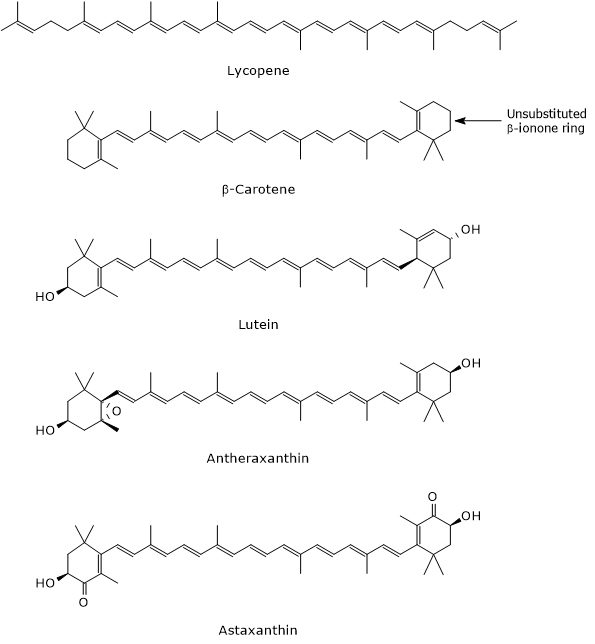
The hydrocarbon chain of carotenoids can undergo hydrogenation, dehydrogenation, introduction of oxygen atoms, cyclization of one or both ends to form an ionone ring, and esterification with fatty acids.[14] These modifications allow for the formation of many different structures, most of which can be described by the chemical formula C40H56On, with 0 ≤ n ≤ 6.[7]
Traditionally, carotenoids have common names related to the biological source from which they were originally extracted, such as β-carotene (from carrot) or astaxanthin (from lobster), although they can also be named using IUPAC nomenclature.[6][15]
Isomerism
Carotenoids contain many double bonds that can exhibit cis–trans isomerism, also known as geometric isomerism. Although, in theory, their double bonds may adopt either a cis or a trans configuration, in nature the trans configuration prevails, as it is thermodynamically more stable due to lower steric hindrance between substituents.[5]
Rotation around single (σ) bonds also allows the formation of conformational isomers. When the double bonds are on the same side with respect to the sigma bond, namely cis relative to the single bond, the conformation is called the s-cis conformation. Conversely, when the double bonds lie on opposite sides, namely trans relative to the single bond, the conformation is called the s-trans conformation.[16]
In nature, acyclic carotenoids occur mainly in the s-trans conformation, as it has the least steric hindrance.[17] When the polyene chain ends with cyclic structures, rotation around the C6–C7 σ bond may lead to the formation of a 6-s-cis isomer, which is sterically less hindered and therefore energetically favored.[18]
It should also be noted that steric hindrance and the size of carotenoid molecules influence their ability to interact with enzymes and to form supramolecular structures.[19]
Most carotenoids possess either a chiral center or a chirality axis, which gives rise to optical isomers. In such cases, to provide an unambiguous name for the molecule, the RS system is used instead of the Fischer-Rosanoff convention, which applies only to carbohydrates and amino acids.[20]
Classification
Carotenoids can be classified according to the presence or absence of oxygen atoms or cyclic structures.[14]
Based on oxygen content, they are divided into xanthophylls and carotenes.
- Carotenes, which are oxygen-free molecules, are composed only of carbon and hydrogen atoms and have the chemical formula C40H56. Examples include α-carotene, β-carotene, δ-carotene, ζ-carotene, phytoene, and lycopene.
- Xanthophylls contain oxygen atoms and have the chemical formula C40H56On, with 1 ≤ n ≤6. They can be further subdivided into:
- hydroxycarotenoids, which contain at least one hydroxyl group, such as α-cryptoxanthin, β-cryptoxanthin, zeaxanthin, and lutein;
- epoxycarotenoids, which have at least one epoxy group, such as antheraxanthin, auroxanthin, and luteoxanthin;
- ketocarotenoids, which contain one or more carbonyl groups, such as astaxanthin and canthaxanthin.[8]
Depending on the presence or absence of cyclic structures, carotenoids can be classified as cyclic or acyclic.
- Cyclic carotenoids contain one or two cyclic structures and are shorter than acyclic carotenoids but exhibit greater steric hindrance. Examples include α-carotene, β-carotene, γ-carotene, and δ-carotene.
- Acyclic carotenoids consist of a linear carbon chain. Examples include lycopene, ζ-carotene, phytoene, and phytofluene.
Finally, there are also uncommon or species-specific carotenoids, such as bixin, capsanthin, and capsorubin.[12]
Solubility
Carotenoids are hydrophobic molecules and therefore soluble in organic solvents, with their solubility varying according to the substituents present.[21] Owing to their hydrophobic nature, they are located within cell membranes. In most cases, xanthophylls have polar groups at the ends of the polyene chain, and when incorporated into lipid bilayers they orient themselves so that their polar groups interact with the polar regions of the membrane, thereby minimizing the system’s energy.[22]
Carotenoids can also access the aqueous environment when associated with proteins to which they bind non-covalently. In plasma, they are transported by lipoproteins.[23]
Colour
The conjugated double-bond system, acting as a chromophore, is responsible for the colour of carotenoids.[14] However, only carotenoids with at least seven conjugated double bonds are coloured; thus, phytofluene, which has five conjugated double bonds, and phytoene, which has three, are colourless.[24]
As the number of conjugated double bonds increases, the colour shifts from yellow to orange to red. For example, lutein, α-cryptoxanthin, and violaxanthin are yellow, α-carotene, β-carotene, and γ-carotene are orange, and lycopene is red.[1]
Role in plants
Carotenoids perform a variety of roles in plants.
They contribute to cell protection against oxidative damage, enhance light absorption, serve as precursors of phytohormones such as abscisic acid, and participate in signaling pathways.[5]
Together with chlorophylls and anthocyanins, a class of flavonoids, they also contribute to the colour of leaves, fruits, vegetables, and grains, a colour that attracts animals and promotes pollination and seed dispersal.[25][26]
Finally, carotenoids can act as repellent agents against phytophagous organisms and pathogens.[27]
Quenching of singlet oxygen
In organisms that carry out oxygenic photosynthesis, carotenoids contribute to protection against damage caused by excessive solar radiation.[28]
When the sunlight arriving at the photosystem II antenna complex exceeds the conversion capacity of the reaction centers, the excess energy may cause chlorophyll to remain in its excited state, which can lead to the formation of its triplet state. The excess energy can then be transferred from chlorophyll to molecular oxygen, resulting in the formation of singlet oxygen, a reactive oxygen species capable of damaging the photosynthetic apparatus and, more generally, nucleic acids, proteins, membrane unsaturated fatty acids, and enzyme cofactors.[29]
Carotenoids can prevent oxidative damage caused by singlet oxygen in two ways.
- They can directly quench singlet oxygen. In this mechanism, the energy is transferred from singlet oxygen to the carotenoid; the oxygen returns to the triplet ground state, while the carotenoid reaches its triplet state and then releases the excess energy as heat. The capacity to quench singlet oxygen increases with the number of conjugated double bonds, reaching its maximum at nine or more.
In vitro studies suggest that monocyclic carotenoids can quench singlet oxygen more efficiently than acyclic ones, and that the presence of carbonyl groups, as in astaxanthin, enhances antioxidant potential compared to carotenoids with hydroxyl groups, likely due to an extended system of conjugated double bonds. It appears that each carotenoid molecule can quench approximately 1,000 singlet oxygen molecules before undergoing degradation.[30] - Carotenoids can prevent the formation of singlet oxygen by absorbing the energy of excited chlorophyll and entering a triplet state themselves. Because the triplet state of carotenoids has a lower energy level than that required to form singlet oxygen, it decays spontaneously to the ground state.[31]
Free radical scavenging activity
Carotenoids are able to scavenge free radicals, such as hydroxyl, peroxyl, superoxide, and nitric oxide radicals. Their action can occur through four different mechanisms.[8][32]
- Electron transfer
Carotenoids can donate an electron to a radical cation, forming a carotenoid radical cation while the radical becomes a stable molecule. The carotenoid radical cation can be regenerated to the parent carotenoid by other cellular antioxidants, such as vitamin E, vitamin C, and glutathione.[33] - Proton transfer
A proton may be transferred from the carotenoid to the radical species, which becomes a neutral molecule, while the carotenoid forms a radical anion.[34] - Addition reactions
Neutralization of free radicals can also occur via addition reactions, as in the case of hydroxyl and peroxyl radicals.[13] - Hydrogen atom transfer
A hydrogen atom can be transferred from the carotenoid to the reactive oxygen species, forming a resonance-stabilized neutral carotenoid radical.[19]
Light-harvesting role
Carotenoids are present in antenna complexes as accessory light-harvesting pigments, mainly associated with antenna proteins.[35]
Thanks to their system of conjugated double bonds, they are able to absorb sunlight in the 400–500 nm range, after which the absorbed energy is transferred to the reaction centers. The absorption spectra of individual carotenoids depend on the length of the conjugated system and the functional groups present.[17]
The main carotene involved in light harvesting is β-carotene, located in the core of photosystems I and II in all organisms that carry out oxygenic photosynthesis. Lutein, violaxanthin, neoxanthin, and zeaxanthin, the main xanthophylls in plants, are bound to the light-harvesting complexes.[36]
Food sources
Humans are not able to synthesize carotenoids. They obtain them from food and store them mainly in the liver and adipose tissue, but also in the lungs, kidneys, brain, and bones, where they perform several functions.[13]
Among all the carotenoids identified, about one hundred have been found in foods consumed by humans. However, within a single food item, there are usually one to six major carotenoids, together with others present only in trace or small amounts.[37]
Fruits and vegetables are the major sources of carotenoids in the human diet. Among vegetables, leafy greens are rich in β-carotene, the most abundant carotenoid in the human diet and in human tissues, and in lutein, followed by violaxanthin and neoxanthin. The carrot root is rich in β-carotene, pumpkin in α-carotene, tomato in lycopene, and peppers in capsanthin and capsorubin. Carotenoids in fruits are highly variable, and their composition often differs between unripe and ripe fruits. Moreover, most carotenoids present in ripe fruits are esterified with fatty acids, whereas fruits that remain green when ripe, such as kiwifruit, show limited or no esterification.[21]
Chicken eggs are also good sources of carotenoids, particularly lutein and zeaxanthin, because laying hens generally consume corn. These carotenoids are responsible for the yellow–orange colour of egg yolk.[38]
Finally, mollusks and crustaceans, together with fish, are major dietary sources of carotenoids produced by microalgae and rarely found in plants, such as astaxanthin, which accumulates in shrimp and contributes to the coloration of salmon.[39]
Food additives
Carotenoids used in the food industry as additives, unlike naturally occurring carotenoids, are relatively unstable. They are susceptible to oxygen and sunlight, auto-oxidation, and dispersion into food ingredients, which facilitates their degradation.[21] Furthermore, at body temperature and food-storage temperatures, added carotenoids are in crystalline form because of their high melting point; to overcome this issue, they are incorporated as oil-in-water emulsions.[40]
Thanks to their colouring power, they are used as colours in foods that lose part of their natural colour due to processing or storage, to standardize product appearance, as in beverages, fruit juices, or sausages, or to intensify colour and improve visual appeal.[41]
Carotenoids are also precursors of compounds responsible for the flavor and aroma of certain foods; therefore, they can be used as flavour enhancers.[42]
In addition, some carotenoids are vitamin A precursors and are used to fortify foods.[43]
It should also be noted that carotenoids, when added to foods, are not powerful antioxidants.[44]
Carotenoids and human health
In humans, carotenoids play important roles.[45]
Their most important role is to act as precursors of vitamin A, which regulates the expression of nearly 700 genes. Not all carotenoids have provitamin A activity; only those with an unsubstituted β-ionone ring do. The most significant is β-carotene which, having two unsubstituted β-ionone rings, can be converted into two molecules of vitamin A. α-Carotene and β-cryptoxanthin, each containing a single unsubstituted β-ionone ring, yield one molecule of vitamin A, and therefore have 50% of the provitamin A activity of β-carotene.[13]
The importance of provitamin A carotenoids is highlighted by the fact that vitamin A deficiency can lead to night blindness and xerophthalmia, delay the growth and regeneration of mucous membranes, increase mortality due to immunological dysfunctions during infectious diseases, and represents the leading cause of childhood blindness in developing countries.[46]
Carotenoids also contribute to human health through mechanisms unrelated to provitamin A activity, such as antioxidant and anti-inflammatory actions and photoprotection. Examples include the following.
- Supplementation with lutein and zeaxanthin, two xanthophylls that accumulate in the macula lutea, has been associated with improved visual function and a reduced risk of progression of age-related macular degeneration. Lutein and zeaxanthin also appear to reduce the risk of cataracts and retinopathies, and lutein may have beneficial effects on cognitive functions.[47]
- Among carotenes, the consumption of lycopene-rich foods has been associated with a reduced incidence of several types of cancer, such as prostate and stomach cancer, as well as a lower cardiovascular risk.[48]
Plasma carotenoids
α-Carotene, β-carotene, lycopene, lutein, β-cryptoxanthin, and zeaxanthin account for more than 90% of the carotenoids present in plasma.[49]
Plasma carotenoid concentrations depend on several factors, including cooking methods, dietary fat intake, and individual differences in lipid digestion and lipid absorption, processes in which bile salts play an essential role. Vitamin A status also influences plasma levels: individuals with low vitamin A stores may have higher conversion rates of provitamin A carotenoids, which can result in lower circulating concentrations.[50][51]
References
- ^ a b c Maoka T. Carotenoids as natural functional pigments. J Nat Med 2020;74(1):1-16. doi:10.1007/s11418-019-01364-x
- ^ a b Sourkes T.L. The discovery and early history of carotene. Bull Hist Chem 2009;34(1):32-37.
- ^ Aizpuru A., González-Sánchez A. Traditional and new trend strategies to enhance pigment contents in microalgae. World J Microbiol Biotechnol 2024;40(9):272. doi:10.1007/s11274-024-04070-3
- ^ de la Rosa L.A., Alvarez-Parrilla E., Gonzàlez-Aguilar G.A. Fruit and vegetable phytochemicals: chemistry, nutritional value, and stability. 1st Edition. Wiley J. & Sons, 2010.
- ^ a b c d Swapnil P., Meena M., Kumar Singh S., Praveen Dhuldhaj U., Harish, Marwal A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr Plant Biol 2021;26: 100203. doi:10.1016/j.cpb.2021.100203
- ^ a b Crupi P., Faienza M.F., Naeem M.Y., Corbo F., Clodoveo M.L., Muraglia M. Overview of the potential beneficial effects of carotenoids on consumer health and well-being. Antioxidants (Basel) 2023;12(5):1069. doi:10.3390/antiox12051069
- ^ a b Desmarchelier C., Borel P. Overview of carotenoid bioavailability determinants: from dietary factors to host genetic variations. Trends Food Sci Technol 2017;69(Part B):270-280. doi:10.1016/j.tifs.2017.03.002
- ^ a b c d Rodriguez-Amaya D.B. Food carotenoids: chemistry, biology, and technology. Hoboken (NJ): Wiley-Blackwell; 2015. doi:10.1002/9781118864364
- ^ Gebhardt B., Sperl R., Carle R., Müller-Maatsch J. Assessing the sustainability of natural and artificial food colorants. J Clean Prod 2020;260;120884. doi:10.1016/j.jclepro.2020.120884
- ^ EFSA Food colours. Last reviewed date: 14 November 2025.
- ^ Olson J.A., Krinsky N.I. Introduction: the colorful, fascinating world of the carotenoids: important physiologic modulators. FASEB J 1995;9(15):1547-50. doi:10.1096/fasebj.9.15.8529833
- ^ a b Britton G., Liaaen-Jensen S., Pfander H. Carotenoids: Handbook. Basel: Birkhäuser; 2004. doi:10.1007/978-3-0348-7836-4
- ^ a b c d Saini R.K., Prasad P., Lokesh V., Shang X., Shin J., Keum Y.S., Lee J.H. Carotenoids: dietary sources, extraction, encapsulation, bioavailability, and health benefits – A review of recent advancements. Antioxidants 2022;11(4):795. doi:10.3390/antiox11040795
- ^ a b c Zia-Ul-Haq M., Dewanjee S., Riaz M. Carotenoids: structure and function in the human body. Springer; 2021. doi:10.1007/978-3-030-46459-2_1
- ^ Yabuzaki J. Carotenoids database: structures, chemical fingerprints and distribution among organisms. Database (Oxford) 2017;2017(1):bax004. doi:10.1093/database/bax004
- ^ Solomons T.W.G., Fryhle C.B., Snyder S.A. Solomons’ organic chemistry. 12th Edition. John Wiley & Sons Incorporated, 2017.
- ^ a b Telegina T.A., Vechtomova Y.L., Aybush A.V., Buglak A.A., Kritsky M.S. Isomerization of carotenoids in photosynthesis and metabolic adaptation. Biophys Rev 2023;15(5):887-906. doi:10.1007/s12551-023-01156-4
- ^ Britton G. Structure and properties of carotenoids in relation to function. FASEB J 1995;9(15):1551-8. doi:10.1096/fasebj.9.15.8529834
- ^ a b Polyakov N.E., Focsan A.L., Gao Y., Kispert L.D. The endless world of carotenoids-Structural, chemical and biological aspects of some rare carotenoids. Int J Mol Sci 2023;24(12):9885. doi:10.3390/ijms24129885
- ^ Prelog V., Helmchen G. Basic principles of the CIP‐system and proposals for a revision. Angew Chem 1982:21(8);567-583. doi:10.1002/anie.198205671
- ^ a b c González-Peña M.A., Ortega-Regules A.E., Anaya de Parrodi C., Lozada-Ramírez J.D. Chemistry, occurrence, properties, applications, and encapsulation of carotenoids-A review. Plants (Basel) 2023;12(2):313. doi:10.3390/plants12020313
- ^ Gruszecki W.I., Strzałka K. Carotenoids as modulators of lipid membrane physical properties. Biochim Biophys Acta 2005;1740(2):108-15. doi:10.1016/j.bbadis.2004.11.015
- ^ Tyssandier V., Choubert G., Grolier P., Borel P. Carotenoids, mostly the xanthophylls, exchange between plasma lipoproteins. Int J Vitam Nutr Res 2002;72(5):300-8. doi:10.1024/0300-9831.72.5.300
- ^ Rodriguez-Amaya D.B., Esquivel P., Meléndez-Martínez A.J. Comprehensive update on carotenoid colorants from plants and microalgae: challenges and advances from research laboratories to industry. Foods 2023;12(22):4080. doi:10.3390/foods12224080
- ^ Archetti, M., Döring T.F., Hagen S.B., Hughes N.M., Leather S.R., Lee D.W., Lev-Yadun S., Manetas Y., Ougham H.J. Unravelling the evolution of autumn colours: an interdisciplinary approach. Trends Ecol Evol 2009;24(3):166-73. doi:10.1016/j.tree.2008.10.006
- ^ Cazzonelli C.I. Carotenoids in nature: insights from plants and beyond. Funct Plant Biol 2011;38(11):833-847.
- ^ Gómez-Sagasti M.T., López-Pozo M., Artetxe U., Becerril J.M., Hernández A., García-Plazaola J.I., Esteban R. Carotenoids and their derivatives: a “swiss army knife-like” multifunctional tool for fine-tuning plant-environment interactions. Environ Exp Bot 2023;207:105229. doi:10.1016/j.envexpbot.2023.105229
- ^ Havaux M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci 1998;3(4):147-151. doi:10.1016/S1360-1385(98)01200-X
- ^ Triantaphylidès C., Havaux M. Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci 2009;14(4):219-28. doi:10.1016/j.tplants.2009.01.008
- ^ Ramel F., Birtic S., Cuiné S., Triantaphylidès C., Ravanat J.L., Havaux M. Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol 2012;158(3):1267-78. doi:10.1104/pp.111.182394
- ^ Pérez-Gálvez A., Viera I., Roca M. Carotenoids and chlorophylls as antioxidants. Antioxidants (Basel) 2020;9(6):505. doi:10.3390/antiox9060505
- ^ Krinsky N.I. Antioxidant functions of carotenoids. Free Radic Biol Med 1989;7(6):617-35. doi:10.1016/0891-5849(89)90143-3
- ^ Ribeiro D., Freitas M., Silva A.M.S., Carvalho F., Fernandes E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem Toxicol 2018;120:681-699. doi:10.1016/j.fct.2018.07.060
- ^ Focsan A.L., Kispert L.D. Radicals formed from proton loss of carotenoid radical cations: a special form of carotenoid neutral radical occurring in photoprotection. J Photochem Photobiol B 2017;166:148-157. doi:10.1016/j.jphotobiol.2016.11.015
- ^ Cogdell R.J., Gillbro T., Andersson P.O., Liu R.S.H., Asato A.E. Carotenoids as accessory light-harvesting pigments. Pure Appl Chem 1994:66(5):1041-1046. doi:10.1351/pac199466051041
- ^ Xu P., Chukhutsina V.U., Nawrocki W.J., Schansker G., Bielczynski L.W., Lu Y., Karcher D., Bock R., Croce R. Photosynthesis without β-carotene. eLife 2020;9:e58984. doi:10.7554/eLife.58984
- ^ Meléndez-Martínez A.J., Mandić A.I., Bantis F., et al. A comprehensive review on carotenoids in foods and feeds: status quo, applications, patents, and research needs. Crit Rev Food Sci Nutr 2022;62(8):1999-2049. doi:10.1080/10408398.2020.1867959
- ^ Ko E.Y., Saini R.K., Keum Y.S., An B.K. Age of laying hens significantly influences the content of nutritionally vital lipophilic compounds in eggs. Foods 2020;10(1):22. doi:10.3390/foods10010022
- ^ Galasso C., Corinaldesi C., Sansone C. Carotenoids from marine organisms: biological functions and industrial applications. Antioxidants (Basel) 2017;6(4):96. doi:10.3390/antiox6040096
- ^ Kirkhus B., Afseth N.K., Borge G.I.A., Grimsby S., Steppeler C., Krona A., Langton M. Increased release of carotenoids and delayed in vitro lipid digestion of high pressure homogenized tomato and pepper emulsions. Food Chem 2019;285:282-289. doi:10.1016/j.foodchem.2019.01.124
- ^ Mezzomo N., Ferreira S.R.S. Carotenoids functionality, sources, and processing by supercritical technology: a review. J Chem 2016;1:Article ID 3164312. doi:10.1155/2016/3164312
- ^ Meléndez-Martínez A.J., Esquivel P., Rodriguez-Amaya D.B. Comprehensive review on carotenoid composition: transformations during processing and storage of foods. Food Res Int 2023;169:112773. doi:10.1016/j.foodres.2023.112773
- ^ Hombali A.S., Solon J.A., Venkatesh B.T., Nair N.S., Peña-Rosas J.P. Fortification of staple foods with vitamin A for vitamin A deficiency. Cochrane Database Syst Rev 2019;5(5):CD010068. doi:10.1002/14651858.CD010068.pub2
- ^ Terao J. Revisiting carotenoids as dietary antioxidants for human health and disease prevention. Food Funct 2023;14:7799-7824. doi:10.1039/d3fo02330c
- ^ Eggersdorfer M., Wyss A. Carotenoids in human nutrition and health. Arch Biochem Biophys 2018;652:18-26. doi:10.1016/j.abb.2018.06.001
- ^ Hodge C., Taylor C. Vitamin A deficiency. [Updated 2023 Jan 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK567744/
- ^ Mrowicka M., Mrowicki J., Kucharska E., Majsterek I. Lutein and zeaxanthin and their roles in age-related macular degeneration-neurodegenerative disease. Nutrients 2022;14(4):827. doi:10.3390/nu14040827
- ^ Khan U.M., Sevindik M., Zarrabi A., et al. Lycopene: food sources, biological activities, and human health benefits. Oxid Med Cell Longev 2021;2021:2713511. doi:10.1155/2021/2713511
- ^ Deming D.M., Erdman Jr J.W. Mammalian carotenoid absorption and metabolism. Pure Appl Chem 1999;71(12):2213-2223.
- ^ Burrows T.L., Williams R., Rollo M., Wood L., Garg M.L., Jensen M., Clare Collins C.E. Plasma carotenoid levels as biomarkers of dietary carotenoid consumption: a systematic review of the validation studies. J Nutr Intermed Metab 2015;2(1-2):15-64. doi:10.1016/j.jnim.2015.05.001
- ^ Böhm V., Lietz G., Olmedilla-Alonso B., et al. From carotenoid intake to carotenoid blood and tissue concentrations – implications for dietary intake recommendations. Nutr Rev 2021;79(5):544-573. doi:10.1093/nutrit/nuaa008