Acidity regulators, also known as pH control agents, are additives used in processed foods to modify or stabilize their acidity or alkalinity, which is measured by the pH value. Their function often overlaps with that of other food additives: some can also act as preservatives, others as emulsifiers, while some help preserve the color of the food to which they are added. In this way, they contribute, along with other factors, to maintaining or improving the organoleptic properties of the product.[1][2]
Like other food additives, acidity regulators are identified both by their chemical name and by the E-numbering system.[3]
According to the scientific evidence currently available, when used in accordance with specific guidelines, acidity regulators are not harmful to human health.[4][5]
Contents
What they are used for
Acidity regulators are employed in a wide range of foods. Their main functions are outlined below.
- pH stabilization.
Some foods require an acidic or neutral environment to maintain their chemical integrity and, consequently, their organoleptic properties. For example, carbonated drinks need acids to prevent changes in taste and composition during storage. - Food preservation.
Acidity helps prevent the growth of harmful microorganisms such as bacteria and mold. This is particularly important in packaged foods and preserves, where a low pH acts as a natural barrier against microbial growth. In this way, acidity regulators can function as preservatives. - Flavor enhancement.
Acidity regulators can balance flavors by adding a sour note that enhances the overall taste. For instance, lactic acid (E270) is used in dairy products and fermented foods to improve flavor. - Support for consistency and quality.
By regulating acidity, these additives can help stabilize the structure of certain foods, such as cheeses, sweets, and ice cream, acting in a way similar to emulsifiers. - Enhancement of antioxidant action.
Some acidity regulators, such as citric acid (E330), though not antioxidants themselves, can enhance the activity of other antioxidants.[1][2][6][7]
Examples of acidity regulators
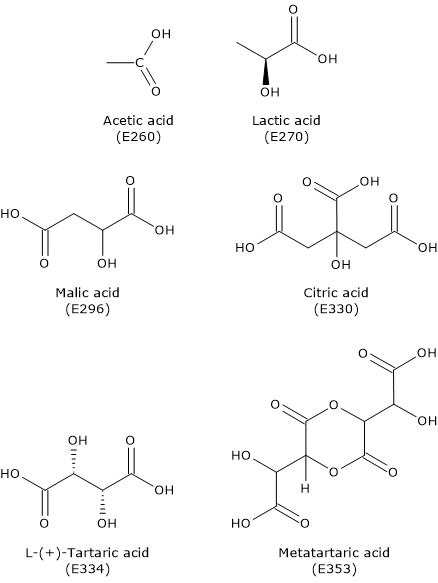
Below is an overview of some acidity regulators included in the list of food additives authorized for use in the European Union under Regulation (EU) No. 1129/2011 of the European Commission, published on November 11, 2011. This list, which amended Annex II of Regulation (EC) No. 1333/2008 of the European Parliament, was later revised in 2013.[8]
| E-number | Additive and salts |
|---|---|
| Acetic acid and its salts | |
| E260 | Acetic acid |
| E261 | Potassium acetate |
| E262 | Sodium acetate |
| E263 | Calcium acetate |
| Lactic acid and its salts | |
| E270 | Lactic acid |
| E325 | Sodium lactate |
| E326 | Potassium lactate |
| E327 | Calcium lactate |
| Citric acid and its salts | |
| E330 | Citric acid |
| E331 | Sodium citrate |
| E332 | Potassium citrate |
| E333 | Calcium citrate |
| E380 | Triammonium citrate |
| Tartaric acid and its salts | |
| E334 | L-(+)-Tartaric acid |
| E335 | Sodium tartrate |
| E336 | Potassium tartrate |
| E337 | Potassium sodium tartrate |
| E353 | Metatartaric acid |
| E354 | Calcium tartrate |
| Malic acid and its salts | |
| E296 | Malic acid |
| E350 | Sodium malate |
| E351 | Potassium malate |
| E352 | Calcium malate |
Health effects
As with other food additives, the safety of acidity regulators is evaluated by the competent authorities on the basis of the scientific literature available at the time of the assessment.[4]
 According to current scientific evidence, when used in accordance with regulations, acidity regulators such as acetic acid (E260), lactic acid, citric acid and tartaric acid (E334–337, E354) have not been associated with adverse health effects at permitted use levels. A 2020 EFSA Panel evaluation found no safety concerns for these compounds at the reported use concentrations.[7]
According to current scientific evidence, when used in accordance with regulations, acidity regulators such as acetic acid (E260), lactic acid, citric acid and tartaric acid (E334–337, E354) have not been associated with adverse health effects at permitted use levels. A 2020 EFSA Panel evaluation found no safety concerns for these compounds at the reported use concentrations.[7]
However, like all additives, they remain subject to periodic safety reassessments.[5][9]
References
- ^ a b Damodaran S., Parkin K. Fennema’s Food Chemistry. 5th Edition. CRC Press, 2017.doi:10.1201/9781315372914
- ^ a b EUFIC What are acidity regulators and why are they added to food. Last Updated: 01 December 2021. https://www.eufic.org/en/whats-in-food/article/acidity-regulators-the-multi-task-players
- ^ Food Standards Agency. Approved additives and E Numbers. Last updated: 16 July 2025.
- ^ a b Bailey R.L. Current regulatory guidelines and resources to support research of dietary supplements in the United States. Crit Rev Food Sci Nutr 2020;60(2):298-309. doi:10.1080/10408398.2018.1524364
- ^ a b EFSA Food additives: EFSA’s new guidance for applicants. Published: 18 July 2012. https://www.efsa.europa.eu/en/press/news/120718a
- ^ Belitz H.-D., Grosch W., Schieberle P. Food Chemistry. 4th Edition. Springer, 2009.
- ^ a b EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings), Younes M., Aquilina G., Castle L., et al. Scientific opinion on the re-evaluation of acetic acid, lactic acid, citric acid, tartaric acid, mono- and diacetyltartaric acid, mixed acetic and tartaric acid esters of mono- and diglycerides of fatty acids (E 472a-f) as food additives. EFSA Journal 2020;18(3):6032, 66 pp. doi:10.2903/j.efsa.2020.6032
- ^ Commission Regulation (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. https://eur-lex.europa.eu/eli/reg/2011/1129/2013-11-21
- ^ EFSA Food additives. Last reviewed date: 18 July 2025.