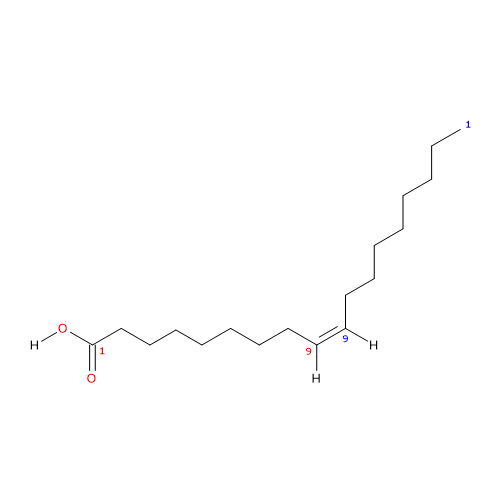
Oleic acid (18 carbon atoms) was recognized in pork fat by Chevreul M.E. in 1823 while the structure was elucidated later by the works of Baruch J. (1894) and Edmed F.G. (1898); it was synthesized for the first time by Noller C.R. et al. in 1934.
It is a monounsaturated fatty acid (one cis double bond, from the methyl end is in omega 9 (ω-9) or n-9, so its shorthand notation is 18:1n-9) fatty acid member of the sub-group called long chain fatty acids (LCFA) (from 14 to 18 carbon atoms).
PROPERTIES
Molecular weight: 282.46136 g/mol
Molecular formula: C18H34O2
IUPAC name: (Z)-octadec-9-enoic acid
CAS registry number: 112-80-1
PubChem: 445639
In the pure form is a colorless or nearly colorless liquid insoluble in water, with boiling point at 286 °C (546.8 °F; 559.15 K) at 100 mm Hg. In the solid state its melting point is at 4 °C (39.2 °F; 312.35 K) (ref. Merck, thirteenth edition; other sources report a melting point at 16 °C (60.8 °F; 289.15 K), likely referred to not pure form available in commerce and containing 7-12% saturated fatty acids, e.g., stearic acid, palmitic acid and also some linoleic acid, etc., unsaturated fatty acids).
OTHER NAMES
- cis-9-Octadecenoic acid
- 9Z-octadecenoic acid
- 9-octadecylenic acid
- 18:1n-9
Synthesis and metabolism of oleic acid
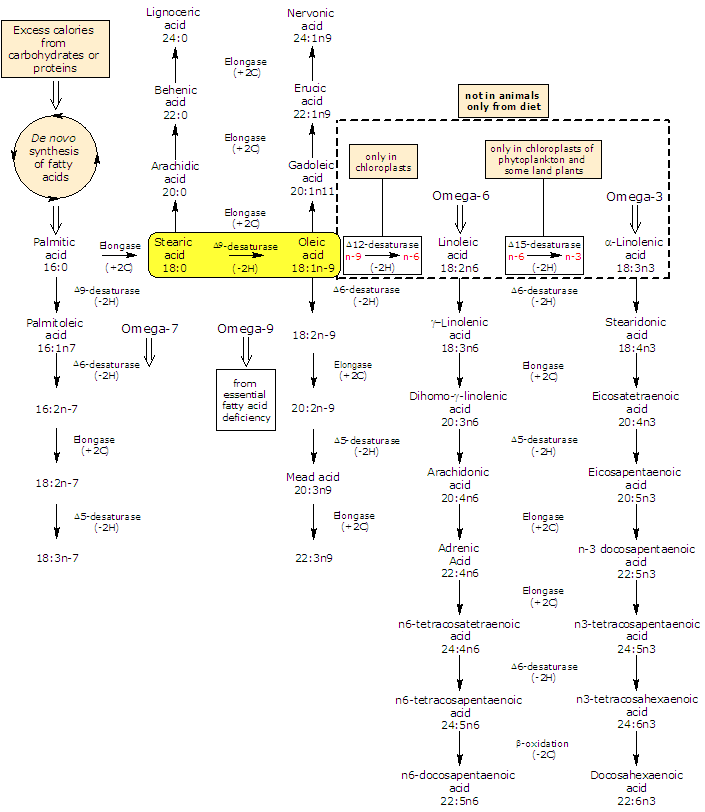
Oleic acid is by far the most widely distributed and abundant fatty acid in nature (few fats known to contain less than 10 percent) where it is mainly found as glycerol ester. It is a Δ9 desaturase product of stearic acid and is the the precursor of most polyunsaturated fatty acids (or PUFA): plants produce both omega-3 polyunsaturated fatty acids and omega-6 polyunsaturated fatty acids from it while animals can elongate and desaturated it to form a variety of omega-9 fatty acids.
The high content is found in olive oil, about 60-70 percent, but is present also in nut oil and almond (abundant), canola oil, sesame oil, cocoa butter, lard and, to a less extent, in palm oil, tallow, safflower oil and soybean oil.
Note: the chief sources of oleic acid in foods must be extra-virgin olive oil, the tastiest cooking oil.
Health benefits
From the health standpoint oleic acid, that is extra-virgin olive oil, when replaced saturated fats lowers total cholesterol and LDL, acronym of Low Density Lipoprotein, levels, bad cholesterol, while raises HDL,acronym of High Density Lipoprotein, levels, good cholesterol, and slows the development of heart disease.
Oleic acid, with linoleic and palmitic acids, is one of the three most abundant fatty acids in triacylglycerols of the fat and plasma lipoproteins.
References
- Akoh C.C. and Min D.B. “Food lipids: chemistry, nutrition, and biotechnology” 3th ed. 2008
- Chow Ching K. “Fatty acids in foods and their health implication” 3th ed. 2008