Glycogen metabolism consists of two phases: a synthetic phase, in which glycogen synthesis occurs, and a degradative phase, known as glycogenolysis, during which the polysaccharide is catabolized.[1]
Glycogenolysis releases glucose when it is required.[2]
In the liver, glycogen serves as a glucose reserve that helps maintain normal blood glucose levels. Its breakdown occurs primarily:
- in the fasted state, e.g. during the nocturnal fast;
- between meals;
- during high-intensity physical activity.[3]
In hepatocytes, glycogenolysis is stimulated by glucagon and adrenaline, inhibited by insulin, and also subject to negative allosteric regulation by glucose.[4]
Glycogen is an important energy source for muscular activity. Therefore, glycogen breakdown occurs during contraction, and only in muscles involved in the activity.
In muscle cells, glycogenolysis is stimulated by adrenaline and regulated by both positive and negative allosteric effectors: AMP and calcium ions act as positive effectors, whereas ATP and glucose 6-phosphate (G6P) act as negative effectors.[5]
Contents
- Steps of glycogenolysis
- Products of glycogenolysis
- Covalent regulation in muscle and liver
- Allosteric regulation in muscle and liver
- References
Steps of glycogenolysis
Glycogenolysis begins with the action of glycogen phosphorylase (EC 2.4.1.1), a homodimeric enzyme that requires pyridoxal 5′-phosphate, a derivative of vitamin B6, as a cofactor.
The enzyme catalyzes the phosphorolytic cleavage of the α-(1,4) glycosidic bond, releasing one glucose residue at a time from the non-reducing ends, that is, the ends with a free 4′-OH group.
This reaction does not consume ATP but instead uses inorganic phosphate (Pi), producing glucose 1-phosphate (G1P).[6]
Glycogen(n glucose residues) + Pi → G1P + Glycogen(n−1 glucose residues)
Note: in the small intestine, pancreatic alpha-amylase (EC 3.2.1.1) catalyzes the hydrolytic cleavage of the α-(1,4) glycosidic bonds of starch, yielding glucose.[7]
In vivo, glycogen phosphorylase catalyzes an irreversible phosphorolysis, a particularly advantageous reaction for skeletal muscle and heart.
The irreversibility of this reaction is ensured by the [Pi]/[G1P] ratio, usually greater than 100. Conversely, under in vitro conditions, the reaction is easily reversible.[8]
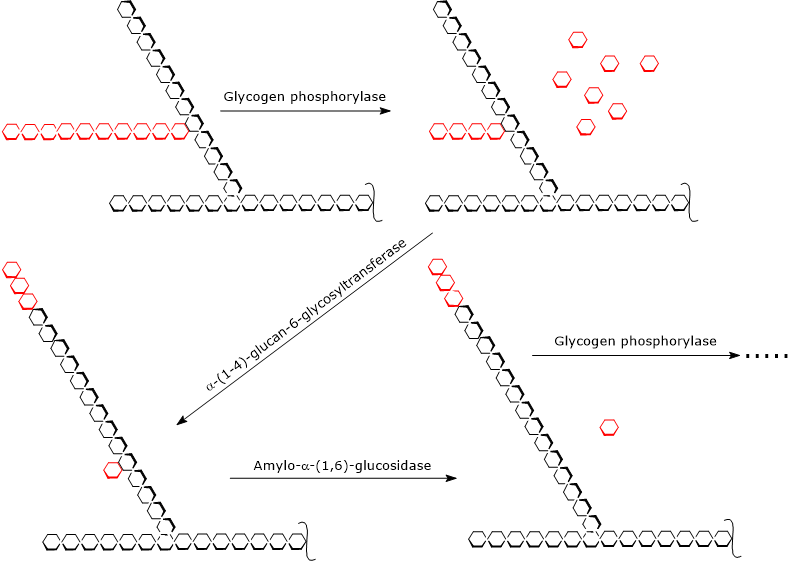
During glycogenolysis, glycogen phosphorylase acts repeatedly on the non-reducing ends of branches until it reaches a glucose residue that is four units away from a branch point. This marks the outer limit of the limit dextrin.[5]
At this stage, two enzymatic activities, located on the same polypeptide chain, complete glycogen degradation: α-(1,4)-glucan-6-glycosyltransferase (EC 2.4.1.25) and amylo-α-(1,6)-glucosidase (EC 3.2.1.33), also known as debranching enzyme.
The first activity transfers three of the remaining four glucose residues from the branch to the non-reducing end of another chain, leaving behind a single glucose residue attached by an α-(1,6) glycosidic bond.
The second activity then hydrolyzes this α-(1,6) glycosidic bond, releasing free glucose and producing an unbranched chain of α-(1,4)-linked glucose units.[9][10]
Once the branch has been removed, glycogen phosphorylase can resume removing glucose residues until it reaches the next limit dextrin.[4]
Products of glycogenolysis
The products of the reactions catalyzed by glycogen phosphorylase and the two activities of the debranching enzyme, α-(1,4)-glucan-6-glycosyltransferase and amylo-α-(1,6)-glucosidase, are:
- glucose 1-phosphate, accounting for about 90% of the released glucose residues;
- a small amount of free glucose (approximately 10%), corresponding to the α-(1,6)-linked residues. In muscle, the activity of hexokinase (EC 2.7.1.1) is so high that any free glucose is rapidly phosphorylated to G6P, thereby becoming metabolically active within the cell;
- a smaller and less branched glycogen molecule.[4]
Metabolic fate of G1P in muscle and liver
Glucose 1-phosphate is a charged molecule and therefore is trapped within the cell.
It is converted to glucose 6-phosphate by the enzyme phosphoglucomutase (EC 5.4.2.2), the same enzyme that also participates in glycogen synthesis by catalyzing the conversion of glucose 6-phosphate to glucose 1-phosphate.
This enzyme catalyzes a reversible reaction, whose direction depends on the relative concentrations of the two metabolites. In this case, the phosphate group is transferred from C1 to C6.[8]
G1P ⇄ G6P
In the muscle and in most other organs and tissues, glucose derived from glycogenolysis enters glycolysis as G6P, thereby bypassing the activation step catalyzed by hexokinase.
Consequently, glycogen phosphorylase, by releasing a pre-activated glucose molecule, saves one ATP molecule.
An ATP molecule is still required for the synthesis of another glycolytic intermediate, fructose 1,6-bisphosphate.
In this way, some of the activation energy invested during glycogen synthesis is conserved.
As a result, the net ATP yield per glucose residue converted to lactate via glycolysis is three ATP instead of two, an energetic advantage for the working muscle.
The overall equation is:
Glycogen(n glucose residues) + 3 ADP + 3 Pi → Glycogen(n−1 glucose residues) + 2 Lactate + 3 ATP
In the liver, glucose 6-phosphate derived from glycogen is dephosphorylated by glucose 6-phosphatase (EC 3.1.3.9) and then released into the bloodstream.
These are the steps involved in the removal of glucose units, in the form of phosphorylated glucose, during hepatic glycogenolysis:[5]
Glycogen(n glucose residues) + Pi → G1P + Glycogen(n−1 glucose residues)
G1P → G6P
G6P + H2O → Glucose + Pi
Overall equation:
Glycogen(n glucose residues) + H2O → Glycogen(n−1 glucose residues) + Glucose
Covalent regulation in muscle and liver
Glycogen breakdown is finely regulated through covalent and/or allosteric modifications of key enzymes, such as phosphorylase kinase (EC 2.7.11.19), glycogen phosphorylase, and phosphorylase a phosphatase (EC 3.1.3.17), also known as protein phosphatase 1 (PP1).[6]
Here, we analyze the effects of two hormones that act through covalent modification of their target proteins:
- adrenaline (epinephrine), produced by the adrenal glands, which acts on muscle, liver, and adipose cells;
- glucagon, produced by the pancreatic α-cells, which acts primarily on hepatocytes and adipocytes.
These hormones, upon binding to their membrane receptors, trigger an identical cascade of intracellular events that amplify their signal by several orders of magnitude, stimulating glycogenolysis and inhibiting glycogen synthesis.[5]
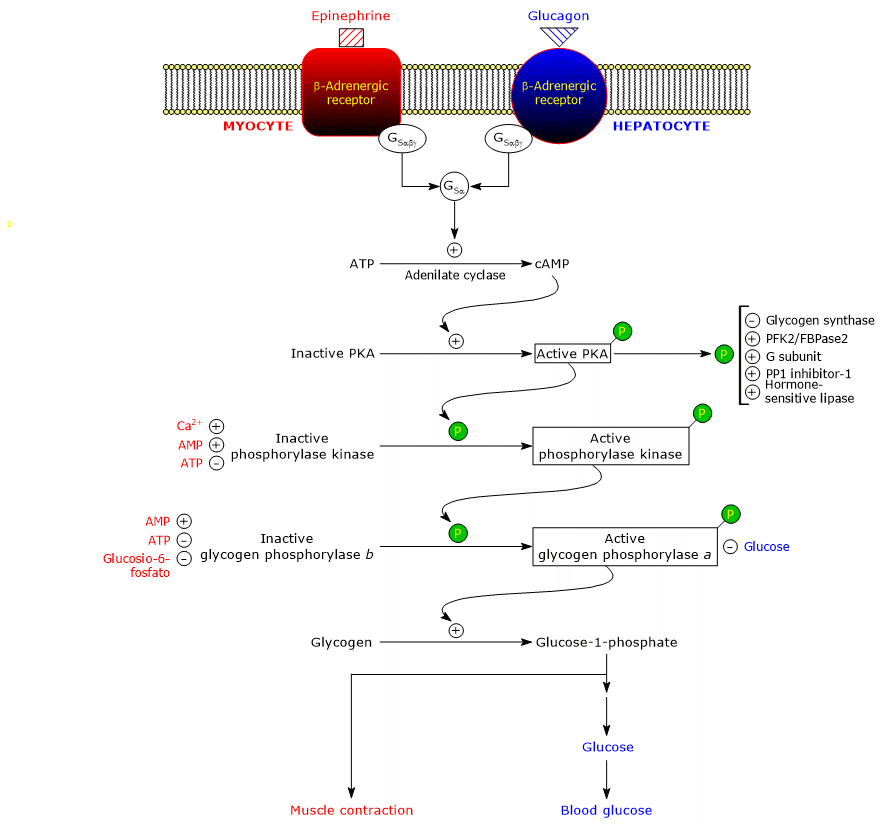
It should be noted that acetylcholine, upon binding to its receptor at the neuromuscular junction, can also trigger a cascade of activations similar to that induced by adrenaline and glucagon.[11]
Below are the proteins involved in this cascade reaction.
β-Adrenergic receptors
The receptors for adrenaline and glucagon are integral membrane proteins characterized by seven transmembrane α-helices.
There are four subtypes of adrenergic receptors: α1, α2, β1 and β2.
In the following discussion, only the β1 and β2 receptors, collectively referred to as β receptors, will be considered, as they act in the same way.[12][13]
β-Adrenergic receptors induce changes in energy metabolism, such as:
- an increase in glycogenolysis in muscle and liver cells;
- an increase in triglyceride breakdown, that is, lipolysis in adipose tissue.[4]
Stimulatory G proteins
The binding of a hormone to its receptor induces a conformational change in the cytosolic portion of the receptor.
This alters its interaction with the next protein in the cascade: the stimulatory guanine nucleotide-binding protein, or more simply, the stimulatory G protein (GS).
It is a heterotrimer composed of three subunits:
- α, which contains the nucleotide-binding site;
- β;
- γ.
In its inactive form, GSαβγ-GDP, the heterotrimer is coupled to β-adrenergic receptors.
The conformational change in the receptor enables it to catalyze the exchange of GDP for GTP on the α subunit of the GS complex.
This exchange leads to the dissociation of the trimer into an inactive βγ dimer and an active GSα-GTP complex, which moves along the inner surface of the plasma membrane.
The GSα-GTP complex remains anchored to the membrane by a covalently linked palmitoyl group and travels until it reaches adenylyl cyclase (EC 4.6.1.1).[13]
Note: the action of GS is similar to that of Ras proteins, another class of G-proteins involved in insulin signal transduction.[14]
Adenylyl cyclase
Adenylyl cyclase is an integral membrane enzyme, whose active site faces the cytosolic side of the plasma membrane.
The interaction between GSα and adenylyl cyclase activates the enzyme which catalyzes the synthesis of cyclic AMP (cAMP) from ATP.
This results in an increase in the intracellular concentration of cAMP.
The stimulatory activity of GSα is self-limiting, since it is a GTPase, that is, it hydrolyzes its bound GTP to GDP, thereby turning itself off.
In its inactive GDP-bound form, GSα dissociates from adenylyl cyclase and reassociates with the Gβγ dimer. Thus, the heterotrimer becomes available again to interact with a new hormone–receptor complex.[13][15]
Protein kinase A
cAMP binds and activates cAMP-dependent protein kinase, also known as protein kinase A (PKA; EC 2.7.11.11).
The inactive form of the enzyme is a tetramer composed of two catalytic subunits and two regulatory subunits.
Each regulatory subunit contains an autoinhibitory domain, a region that occupies the substrate-binding site of each catalytic subunit.
The binding of two cAMP molecules to the two sites on each regulatory subunit induces a conformational change that causes dissociation of the tetramer, releasing the two catalytic subunits as active enzymes.[16]
The active form of PKA catalyzes the phosphorylation of several proteins, thereby activating or inhibiting them, such as:
- glycogen synthase (EC 2.4.1.11), inhibited;
- hormone-sensitive lipase (EC 3.1.1.79), activated;
- phosphofructokinase 2/fructose-2,6-bisphosphatase (EC 2.7.1.105 and EC 3.1.3.46, respectively), activated;
- inhibitor-1 and the glycogen-binding (G) subunit of PP1, activated;
- phosphorylase kinase, activated.[11]
cAMP has a very short half-life: it is hydrolyzed to AMP, which has no second messenger activity, in the reaction catalyzed by cyclic nucleotide phosphodiesterase (EC 3.1.4.53).
Caffeine and theophylline, two methylxanthines found in coffee and tea, respectively, inhibit phosphodiesterase, thereby increasing the half-life of cAMP and enhancing its effects.[8][17]
Phosphorylase kinase
The next step in the cascade is catalyzed by phosphorylase kinase.
This enzyme is composed of four different subunits, each present in four copies, forming a complex referred to as (αβγδ)4.
γ subunits possess catalytic activity, whereas α, β and δ are regulatory.
α and β subunits are phosphorylated when the enzyme transitions from the inactive to the active state.[18]
The δ subunit, also known as calmodulin, is a calcium-binding regulatory protein.
Calmodulin is found in many other enzymes, where it functions as a calcium ion sensor, responding to changes in intracellular calcium concentration and modulating the activity of associated proteins.[4]
Phosphorylase kinase exists in two isoforms: one expressed in the liver and the other in skeletal and cardiac muscle.
They differ in their α and γ subunits, which are encoded by distinct genes.[19]
One of its main target is glycogen phosphorylase, an enzyme that exists as isoenzymes in different tissues, and in two conformational states in dynamic equilibrium, referred to as:
- T (tense or taut), the less active form;
- R (relaxed), the more active form, able to bind glycogen even when phosphorylated.
Phosphorylase kinase phosphorylates a single serine residue (Ser14) on each of the two subunits of glycogen phosphorylase, which is predominantly in the T state.
This conversion produces the active form, mainly in the R state, triggering glycogen breakdown.
The phosphorylated enzyme, glycogen phosphorylase a, is the active form, whereas the non-phosphorylated enzyme, glycogen phosphorylase b, is less active.
Both forms are also subject to allosteric regulation.[11]
In the muscle, glycogenolysis releases glucose 1-phosphate which is metabolized locally to supply energy for muscle contraction, and the fight-or-flight response triggered by adrenaline.[19]
In the liver, glucagon stimulates the release of glucose into the bloodstream to counteract hypoglycemia.[5]
Regulation by PP1 and PKA
Once the stressful situation ends, PP1 removes phosphate groups from phosphorylase kinase and glycogen phosphorylase a, thereby converting them to their inactive forms.
The enzyme also dephosphorylates glycogen synthase.[8]
PP1 is composed of a catalytic subunit, which has low catalytic efficiency and low affinity for glycogen, and the aforementioned G subunit, which belongs to a family of glycogen-targeting proteins that anchor other enzymes to glycogen particles.
Notably, phosphorylase kinase, glycogen phosphorylase, and glycogen synthase are all bound to glycogen particles through proteins of this family.[20]
PP1 is also inhibited by another regulatory protein known as inhibitor-1 of PP1.[21]
PKA phosphorylates:
- the G-subunit, which, in its phosphorylated form, cannot bind the catalytic subunit of PP1 and therefore prevents PP1 from dephosphorylating its glycogen-associated targets (conversely, phosphorylation of the G subunit induced by insulin, at different amino acid residues, promotes binding to the catalytic subunit of PP1);
- inhibitor-1, which, when phosphorylated, inhibits PP1 activity.
Therefore, the binding of a hormone to its receptor initiates a cascade that ultimately inhibits PP1 activity. This keeps both glycogen phosphorylase and glycogen synthase in their phosphorylated states, the former activated and the latter inhibited. In this way, the metabolism of carbohydrates is optimized.[5]
Allosteric regulation in muscle and liver
Glycogenolysis is also regulated by both positive and negative allosteric effectors, acting on three enzymes: muscle phosphorylase kinase, hepatic and muscle glycogen phosphorylase, and PP1, through mechanisms whose structural complexity has been well characterized.[22]
Muscle phosphorylase kinase
Enzyme activity is regulated by two positive allosteric effectors, calcium ions and AMP, and one negative effector, ATP.
An increase in intracellular calcium ion concentration serves as the signal for muscle contraction. Once released from the sarcoplasmic reticulum, calcium binds to calmodulin (the δ subunit of the enzyme), thereby activating it.
AMP accumulates in muscle during intense contraction as a result of ATP consumption and binds to, and activates, the enzyme.
Conversely, when ATP concentration is high, i.e., when the muscle is not contracting, it binds to the allosteric site for AMP, thereby inactivating the kinase.[11]
Hepatic phosphorylase kinase
Some hormones can act both by triggering covalent modifications of target proteins and by promoting the release of calcium ions from the endoplasmic reticulum.
In the liver, phosphorylase kinase is regulated by hormones that induce calcium release. Examples include vasopressin and adrenaline, the latter when it binds to α1 receptors.
Upon binding to these receptors, adrenaline activates a G protein that stimulates phospholipase C-β, which in turn converts phosphatidylinositol-4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG).
IP3 causes the release of calcium ions from the endoplasmic reticulum. The calcium ions, by binding to the δ subunit of phosphorylase kinase, lead to its activation.[11]
Muscle glycogen phosphorylase
Muscle glycogen phosphorylase b is activated in the presence of high concentrations of AMP, which, by binding to a specific nucleotide-binding site, alters the quaternary structure of the enzyme, shifting the allosteric equilibrium toward the active R state of the b form.
Conversely, ATP and G6P, which compete with AMP for the same nucleotide-binding site, act as negative allosteric effectors, shifting the equilibrium toward the inactive T state of the b form.
Muscle glycogen phosphorylase a is active regardless of AMP, ATP, or G6P levels.
In resting muscle, nearly all glycogen phosphorylase exists in the inactive b form.[4][23]
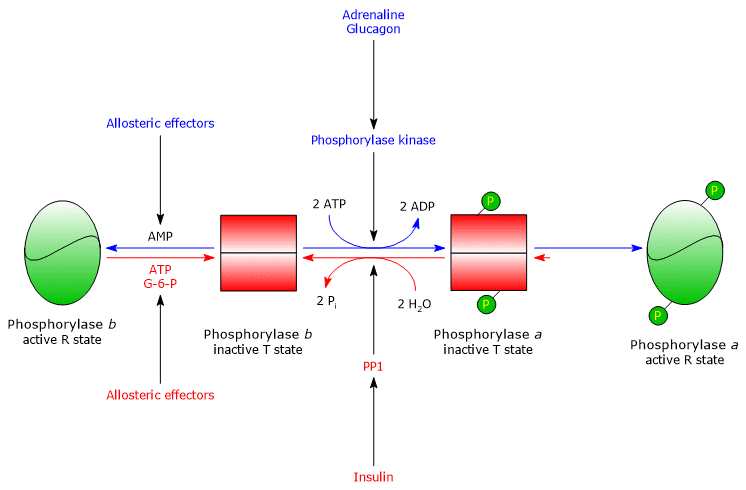
The combined covalent and allosteric regulation of this enzyme ensures that intracellular glucose levels are finely controlled.
- If a cell with an adequate energy charge receives a hormonal signal that triggers the activation cascade, glycogen phosphorylase b, inhibited by ATP and G6P, remains in the T state until the energy charge decreases.
- If the cellular energy charge is low, glycogen phosphorylase b, activated by AMP, initiates glycogenolysis even in the absence of the hormonal stimulus that converts it to the active a form.[5]
Allosteric regulation of PP1
PP1 activity is also modulated allosterically by glucose 6-phosphate, which activates the enzyme when the cellular energy charge is low.[21]
Hepatic glycogen phosphorylase
In the liver, the allosteric regulation of glycogen phosphorylase occurs through mechanisms distinct from those in muscle.
- The first difference concerns the response to AMP: hepatic glycogen phosphorylase b is not activated by AMP.
- The second difference involves glycogen phosphorylase a, which is inhibited by glucose. Glucose acts as a competitive inhibitor, shifting the allosteric equilibrium toward the inactive T state of the a form.[4][24]
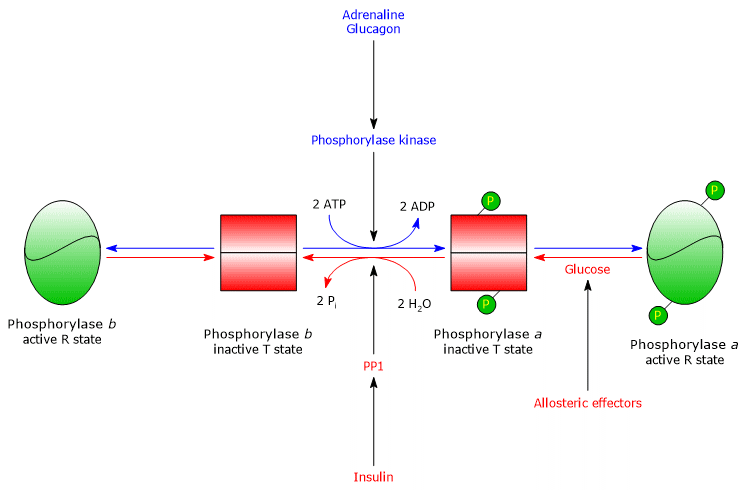
In the liver, the purpose of glycogenolysis is to supply glucose to other tissues when blood glucose levels are low.
When blood glucose returns to normal, its concentration in hepatocytes rises, and glucose binds to glycogen phosphorylase a. This binding induces a conformational change that exposes the phosphorylated serine residues to PP1, which inactivates the enzyme through dephosphorylation.
Thus, the glucose-binding site of hepatic glycogen phosphorylase allows the enzyme to act as a blood glucose sensor, responding appropriately to changes in glucose concentration.
Ultimately, the hepatic isoenzyme responds only to glucose, not to AMP, that is, not to the cellular energy charge, which is noteworthy because fatty acids, rather than glucose, are the primary energy source for the liver.[5]
References
- ^ Patino S.C., Orrick J.A. Biochemistry – Glycogenolysis. [Updated 2024 Jan 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK549820/
- ^ Neoh G.K.S., Tan X., Chen S., Roura E., Dong X., Gilbert R.G. Glycogen metabolism and structure: a review. Carbohydr Polym 2024;346:122631. doi:10.1016/j.carbpol.2024.122631
- ^ Adeva-Andany M.M., González-Lucán M., Donapetry-García C., Fernández-Fernández C., Ameneiros-Rodríguez E. Glycogen metabolism in humans. BBA Clin 2016;27;5:85-100. doi:10.1016/j.bbacli.2016.02.001
- ^ a b c d e f g Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 8th Edition. W.H. Freeman and Company, 2021.
- ^ a b c d e f g h Stipanuk M.H., Caudill M.A. Biochemical, physiological, and molecular aspects of human nutrition. 4th Edition. St. Louis: Elsevier, 2018.
- ^ a b Berg J.M., Tymoczko J.L., Gregory J.G. Jr, Stryer L. Biochemistry. 9th Edition. W.H. Freeman and Company, 2019.
- ^ Janeček Š., Svensson B., MacGregor E.A. α-Amylase: an enzyme specificity found in various families of glycoside hydrolases. Cell Mol Life Sci 2014;71(7):1149-70. doi:10.1007/s00018-013-1388-z
- ^ a b c d Garrett R.H., Grisham C.M. Biochemistry. 7th Edition. Brooks/Cole, Cengage Learning, 2023.
- ^ Gillard B.K., Nelson T.E. Amylo-1,6-glucosidase/4-alpha-glucanotransferase: use of reversible substrate model inhibitors to study the binding and active sites of rabbit muscle debranching enzyme. Biochemistry 1977;16(18):3978-87. doi:10.1021/bi00637a007
- ^ Guan H., Chen H., Geng H., Ma R., Liu Z., Wang Y., Chen Y., Yan K. Molecular architecture and catalytic mechanism of human glycogen debranching enzyme. Nat Commun 2025;16(1):5962. doi:10.1038/s41467-025-61077-6
- ^ a b c d e Heilman D., Woski S., Voet D., Voet J.G., Pratt C.W. Fundamentals of biochemistry: life at the molecular level. 6th Edition. Wiley, 2023.
- ^ Brueckner F., Piscitelli C.L., Tsai C.J., Standfuss J., Deupi X., Schertler G.F. Structure of β-adrenergic receptors. Methods Enzymol 2013;520:117-51. doi:10.1016/B978-0-12-391861-1.00006-X
- ^ a b c Weis W.I., Kobilka B.K. The molecular basis of G protein-coupled receptor activation. Annu Rev Biochem 2018;87:897-919. doi:10.1146/annurev-biochem-060614-033910
- ^ Mozzarelli A.M., Simanshu D.K., Castel P. Functional and structural insights into RAS effector proteins. Mol Cell 2024;84(15):2807-2821. doi:10.1016/j.molcel.2024.06.027
- ^ Qi C., Sorrentino S., Medalia O., Korkhov V.M. The structure of a membrane adenylyl cyclase bound to an activated stimulatory G protein. Science 2019;364(6438):389-394. doi:10.1126/science.aav0778
- ^ Søberg K., Skålhegg B.S. The Molecular basis for specificity at the level of the protein kinase a catalytic subunit. Front Endocrinol (Lausanne) 2018;9:538. doi:10.3389/fendo.2018.00538
- ^ Wombacher H. Theophylline effect on the cyclic AMP degrading multienzyme sequence. Biochem Pharmacol 1982;31(21):3441-6. doi:10.1016/0006-2952(82)90624-4
- ^ Thompson J.A., Carlson G.M. The regulatory α and β subunits of phosphorylase kinase directly interact with its substrate, glycogen phosphorylase. Biochem Biophys Res Commun 2017;482(2):221-225. doi:10.1016/j.bbrc.2016.11.044
- ^ a b Migocka-Patrzałek M., Elias M. Muscle glycogen phosphorylase and its functional partners in health and disease. Cells 2021;10(4):883. doi:10.3390/cells10040883
- ^ Terrak M., Kerff F., Langsetmo K., Tao T., Dominguez R. Structural basis of protein phosphatase 1 regulation. Nature 2004;429(6993):780-4. doi:10.1038/nature02582
- ^ Peti W., Nairn A.C., Page R. Structural basis for protein phosphatase 1 regulation and specificity. FEBS J 2013;280(2):596-611. doi:10.1111/j.1742-4658.2012.08509.x
- ^ Brushia R.J., Walsh D.A. Phosphorylase kinase: the complexity of its regulation is reflected in the complexity of its structure. Front Biosci 1999;4:D618-41. doi:10.2741/brushia
- ^ Johnson L.N. Glycogen phosphorylase: control by phosphorylation and allosteric effectors. FASEB J 1992;6(6):2274-82. doi:10.1096/fasebj.6.6.1544539
- ^ Johnson L.N., Barford D. The effects of phosphorylation on the structure and function of proteins. Annu Rev Biophys Biomol Struct 1993;22:199-232. doi:10.1146/annurev.bb.22.060193.001215