Carbohydrate ingestion can improve endurance capacity and performance.
The ingestion of different types of carbohydrates, which use different intestinal transporters, can:
- increase total carbohydrate absorption;
- increase exogenous carbohydrate oxidation;
- and therefore improve performance.
Glucose and fructose
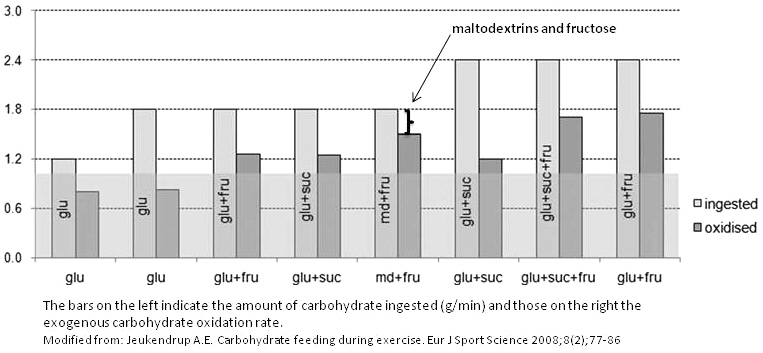
When a mixture of glucose and fructose is ingested, in the analyzed literature, respectively 1.2 and 0.6 g/min, ratio 2:1, for total carbohydrate intake rate to 1.8 g/min, there is less competition for intestinal absorption compared with the ingestion of an iso-energetic amount of glucose or fructose, two different intestinal transporters being involved. Furthermore, fructose absorption is stimulated by the presence of glucose.
This can:
- contribute to a faster rate of the absorption of monosaccharides;
- increase the availability of exogenous carbohydrates in the bloodstream;
- cause the higher exogenous carbohydrate oxidation rates in fructose plus glucose combination compared to high glucose intake alone.
The combined ingestion of glucose and fructose allows to obtain exogenous carbohydrate oxidation rate around 1,26 g/min, therefore, higher than the rate reported with glucose alone (1g/min), also in high concentration.
The observed difference (+0,26 g/min) can be fully attributed to the oxidation of ingested fructose.
Sucrose and glucose
The ingestion of sucrose and glucose, in the same conditions of the ingestion of glucose and fructose (therefore, respectively 1.2 and 0.6 g/min, ratio 2:1, for total carbohydrate intake rate to 1.8 g/min), gives similar results.
Glucose, sucrose and fructose
Very high oxidation rates are found with a mixture of glucose, sucrose, and fructose, in the analyzed literature, respectively 1.2, 0.6 and 0.6 g/min, ratio 2:1:1, for total carbohydrate intake rate to 2.4 g/min; however, note the higher amounts of ingested carbohydrates.
Maltodextrin and fructose
High oxidation rates are also observed with combinations of maltodextrin and fructose, in the same conditions of the ingestion of glucose plus fructose, therefore, respectively 1.2 and 0.6 g/min, ratio 2:1, for total carbohydrate intake rate to 1.8 g/min.
Such high oxidation rates can be achieved with carbohydrates ingested in a beverage, in a gel or in a low-fat, low protein, low-fiber energy bar.
The best combination of carbohydrates ingested during exercise seems to be the mixture of maltodextrin and fructose in a 2:1 ratio, in a 5% solution, and in a dose around 80-90 g/h.
Why?
- This mixture has the best ratio between amount of ingested carbohydrates and their oxidation rate and it means that smaller amounts of carbohydrates remain in the stomach or gut reducing the risk of gastrointestinal complication/discomfort during prolonged exercise (see brackets grafa in the figure).
- A solution containing a combination of multiple transportable carbohydrates and a carbohydrate content not exceeding 5% optimizes gastric emptying rate and improves fluid delivery.
Example of a 5% carbohydrate solution containing around 80-90 g of maltodextrin and fructose in a 2:1 rate; ingestion time around 1 h.
- 1.5 L solution: 80 g of carbohydrates, including around 55 g of maltodextrin and around 25 of fructose.
- 1.8 L solution: 90 g of carbohydrates, including 60 g of maltodextrin and 30 of fructose.
Conclusion
During prolonged exercise, when high exogenous carbohydrate oxidation rates are needed, the ingestion of multiple transportable carbohydrates is preferred above that of large amounts of a single carbohydrate.
The best mixture seems to be maltodextrin and , in a 2:1 ratio, in a 5% concentration solution, and at ingestion rate of around 80-90 g/h.
References
- Burke L.M., Hawley J.A., Wong S.H.S., & Jeukendrup A. Carbohydrates for training and competition. J Sport Sci 2011;29:Sup1,S17-S27. doi:10.1080/02640414.2011.585473
- Jentjens R.L.P.G., Moseley L., Waring R.H., Harding L.K., and Jeukendrup A.E. Oxidation of combined ingestion of glucose and fructose during exercise. J Appl Physiol 2004:96;1277-1284. doi:10.1152/japplphysiol.00974.2003
- Jentjens R.L.P.G., Venables M.C., and Jeukendrup A.E. Oxidation of exogenous glucose, sucrose, and maltose during prolonged cycling exercise. J Appl Physiol 2004:96;1285-1291. doi:10.1152/japplphysiol.01023.2003
- Jeukendrup A.E. Carbohydrate feeding during exercise. Eur J Sport Sci 2008:2;77-86. doi:10.1080/17461390801918971
- Jeukendrup A.E. Nutrition for endurance sports: marathon, triathlon, and road cycling. J Sport Sci 2011:29;sup1, S91-S99. doi:10.1080/02640414.2011.610348