Palmitoleic acid (16 carbon atoms) was noticed first in 1854 by Hofstädter P.G. in sperm whale oil and named physetoleic acid.
In 1906 Bull H. discovered its molecular composition, at the time when Lewkowitsch gave the present name.
The structure was established in 1925 by Armstrong E.F. et al.
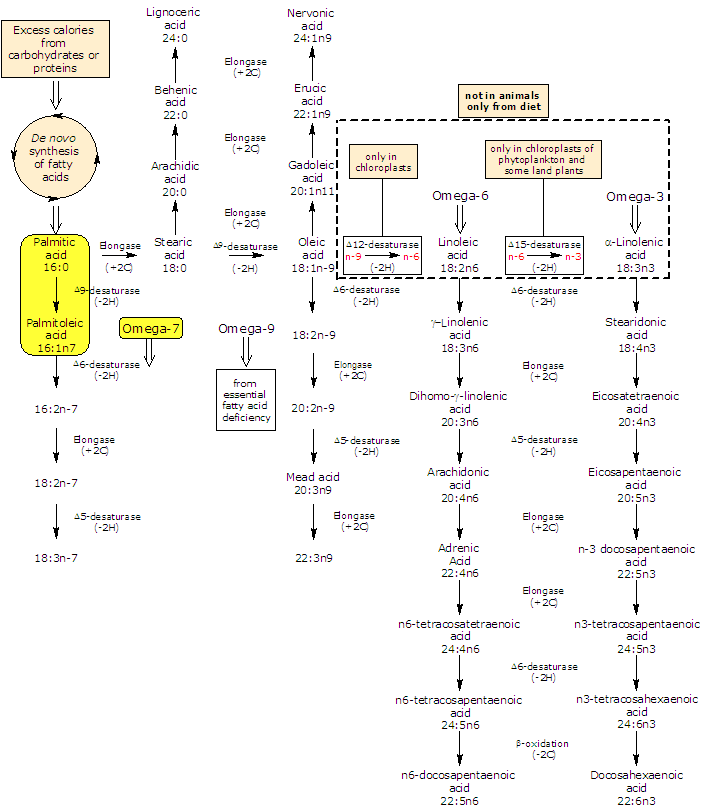
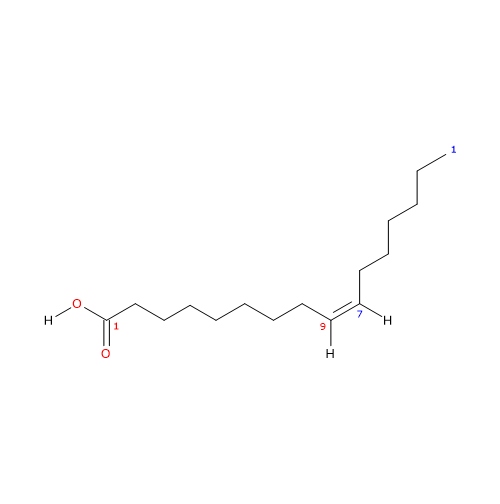
It belongs to the group of unsaturated fatty acids, having one cis double bond, from the methyl end in omega-7 (ω-7) or n-7 position, and its shorthand notation is 16:1n-7. It is also a member of the sub-group called long chain fatty acids (LCFA) (from 14 to 18 carbon atoms).
Both in plants and animals it is produced de novo by the Δ9 desaturation of palmitic acid.
PROPERTIES
Molecular weight: 254.4082 g/mol
Molecular formula: C16H30O2
IUPAC name: (Z)-hexadec-9-enoic acid
CAS registry number: 373-49-9
PubChem: 445638
In purified form its melting point is from -0.5 to +0.5 °C (31.1-32.9 °F; 272.65-273.65 K) and boiling point at 140-141 °C (284-285.8 °F; 413.15-414.15 K) at 5 mm Hg.
OTHER NAMES
zoomaric acid
cis-9-hexadecenoic acid
palmitolinoleic acid
(9Z)-hexadecenoic acid
(Z)-hexadec-9-enoic acid
(9Z)-hexadec-9-enoic acid
cis-delta(9)-hexadecenoic acid
16:1n-7
Food sources of palmitoleic acid
It occurs as glycerol ester mainly in animal fats, particularly in fish and marine mammals, but also in vegetable oils; in the latter sources it is abundant in Roureopsis obliquifoliata, Macadamia ternifolia, Hippophae rhamnoides, Asclepis syriaca seed oils, respectively 32, 20, 16-22, 10 % of the total fatty acids.
In humans is a common component of triglycerides in adipose tissues and is present in higher concentrations in the liver.
A new lipokine
A work of Cao H. et al. (2008) pointed out that adipose tissue communicates with other organs also by palmitoleic acid (so it is a lipokine); in particular researchers demonstrated that this fatty acid strongly stimulates muscle and liver insulin action. This further underscore the highly interconnection between fat metabolism and glucose homeostasis.
References
- Akoh C.C. and Min D.B. “Food lipids: chemistry, nutrition, and biotechnology” 3th ed. 2008
- Cao H., Gerhold K., Mayers J.R., Wiest M.M., Watkins S.M., Hotamisligil G.S. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008; 134:933-944. doi:10.1016/j.cell.2008.07.048
- Chow Ching K. “Fatty acids in foods and their health implication” 3th ed. 2008
- Olefsky J.M. Fat Talks, Liver and Muscle Listen. Cell 2008; 134:914-916. doi:10.1016/j.cell.2008.09.001