gamma-Linolenic acid or γ-linolenic acid or GLA (18 carbon atoms), from the Latin linon, meaning flax, plus oleic, meaning oil or olive oil, was isolated by Heiduschka A. and Luft K. in 1919 from seed oil of Oenothera biennis (evening primrose).
The structure was first proposed by Eibner A. and Luft K. in 1927, and later confirmed by Riley J.P. in 1949.
GLA was synthesized in 1961 by Osbond J.M. et al.
It is a polyunsaturated fatty acid (PUFA) with three cis (Z) double bounds (the first one from the methyl end is in omega-6 (ω-6) or n-6 so in shorthand 18:3n-6) member of the sub-group called long chain fatty acids (LCFA), from 14 to 18 carbon atoms.
PROPERTIES
Molecular weight: 278.4296 g/mol
Molecular formula: C18H30O2
IUPAC name: (6Z,9Z,12Z)-octadeca-6,9,12-trienoic acid
CAS registry number: 506-26-3
PubChem: 5280933
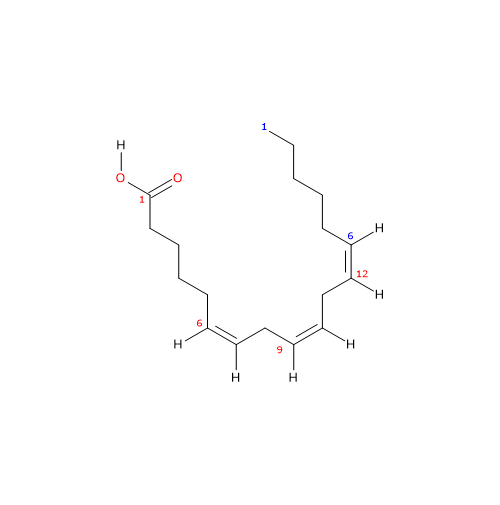
In purified form its melting point is from -11.3 (11.66 °F; 261.85 K) to -11 °C (12.2 °F; 262.15 F) and boiling point at 125 °C (257 °F; 398.15 K) at 0.05 mm Hg.
gamma-Linolenic acid is an isomer of alpha-linolenic acid.
OTHER NAMES
GLA
cis-6,cis-9,cis-12-octadecatrienoic acid
gamolenic acid
18:3n-6
Synthesis and metabolism of gamma-linolenic acid
GLA is produced in lower plants and animals from linoleic acid in a reaction catalyzed by Δ6-desaturase.
In turn, it may be elongated to dihomo-gamma-linolenic acid (DGLA) by an elongase (the enzyme catalyzes the addition of two carbon atoms from glucose metabolism to lengthen the fatty acid chain).
Δ5-desaturase metabolizes dihomo-gamma-linolenic acid to arachidonic acid.
So, by an alternating sequence of Δ6-desaturation, chain elongation, and Δ5-desaturation linoleic acid can be converted to arachidonic acid. The rate limiting enzymes in the conversion of linoleic acid to arachidonic acid are Δ6- and Δ5-desaturase.
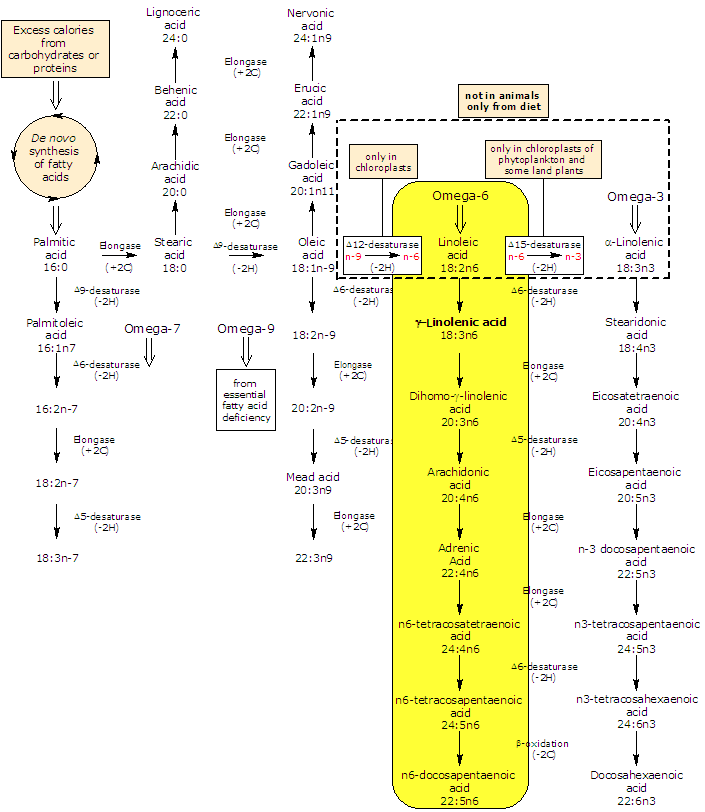
In most tissues Δ5-desaturase has a low activity; for this reason, a small fraction of GLA, or dihomo-γ-linolenic acid from elongation of gamma-linolenic acid, is converted to arachidonic acid. For this metabolic reason, dihomo-gamma-linolenic acid is what accumulates in tissue and cell type after gamma-linolenic acid diet supplementation.
Health benefits of GLA
Bypassing the Δ6-desaturation step, GLA is rapidly elongated to dihomo-γ-linolenic acid, so increasing its concentration.
DGLA is:
- the precursor for the biosynthesis of PGE1 and 15-HETrE which in vivo attenuate inflammatory and proliferative processes;
- it compete with arachidonic acid for incorporation into membrane phospholipids, diminishing the amount of arachidonic acid, and so contributing to the maintenance of the normal fluidity of the cell membrane.
But the biological efficacy of ingested GLA depends on dose per day.
Food sources of gamma-linolenic acid
GLA occurs as glycerol ester in many seed oils from several plant families.
Three major source are:
- Borage (Borago officinalis L.) of the Boraginaceae, the most concentrated form, from 20%-27% of the total fatty acids. Commonly in the Mediterranean region in North Africa;
- Evening primrose (Oenothera biennis L.) of the Onagraceae, good source, from 7% to 14%. It grows in North America;
- Black currant (Ribes nigrum) of the Saxifragaceae, another good source, from 15% to 19%. It grows mainly in Europe;
- Other as Cannabis sativa of the Cannabaceae, Minuartia laricifolia of the Caryophyllaceae, and several microorganisms and fungi.
References
- Akoh C.C. and Min D.B. “Food lipids: chemistry, nutrition, and biotechnology” 3th ed. 2008
- Chow Ching K. “Fatty acids in foods and their health implication” 3th ed. 2008
- Fan Y.Y. and Chapkin R.S. Importance of dietary gamma-linolenic acid in human health and nutrition. J Nutr 1998;128:1411-1414. doi:10.1093/jn/128.9.1411