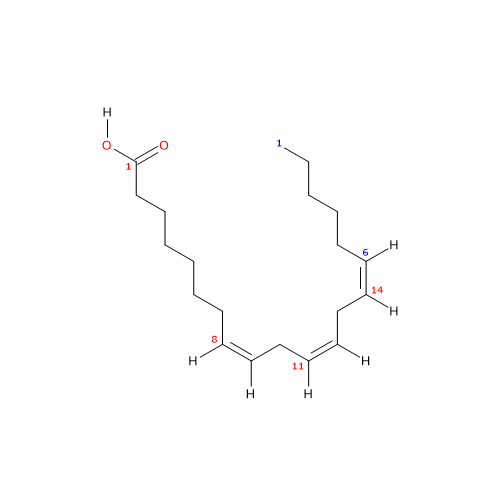
Dihomo-gamma-linolenic acid or dihomo-γ-linolenic acid or DGLA (18 carbon atoms) is a polyunsaturated fatty acid (PUFA) with three cis (Z) double bounds (the first one from the methyl end is in omega-6 (ω-6) or n-6, so in shorthand 20:3n-6) member of sub-group called very long chain fatty acids (VLCFA), from 20 carbon atoms onwards.
PROPERTIES
Molecular weight: 306.48276 g/mol
Molecular formula: C20H34O2
IUPAC name: (8Z,11Z,14Z)-icosa-8,11,14-trienoic acid
CAS registry number: 1783-84-2
PubChem: 5280581
OTHER NAMES
DGLA
bishomo-gamma-linolenic acid
8,11,14-eicosatrienoic acid
8,11,14-all-cis-eicosatrienoic acid
cis-8,11,14-eicosatrienoic acid
20:3n-6
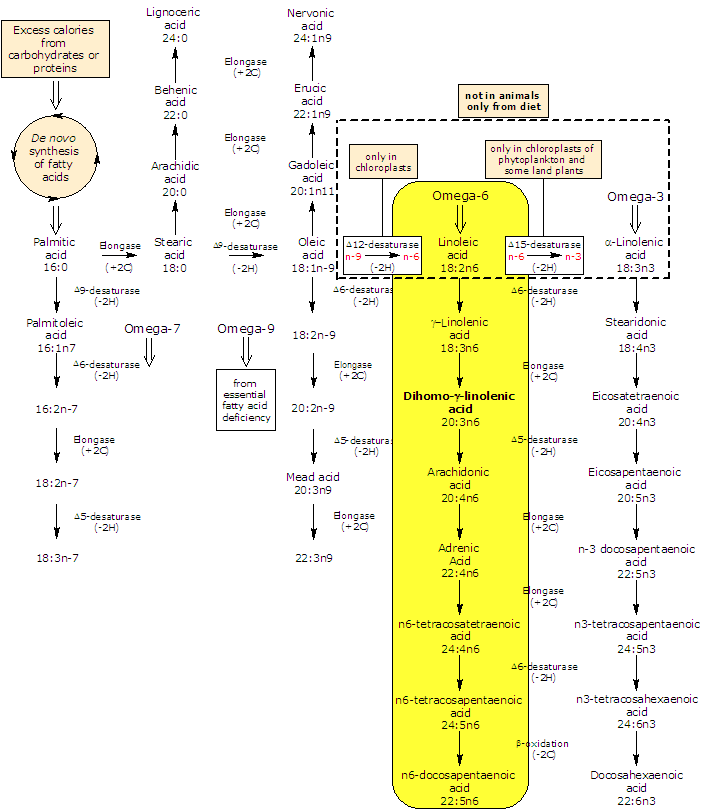
Synthesis and metabolism of dihomo-gamma-linolenic acid
Dihomo-gamma-linolenic acid is produced from gamma-linolenic acid in an elongation reaction catalyzed by an elongase (it catalyzes the addition of two carbon atoms from glucose metabolism to lengthen the fatty acid chain).
It may be further desaturated in very limited amount to arachidonic acid, in a reaction catalyzed by the rate limiting Δ5-desaturase.
DGLA and prostaglandins
Dihomo-gamma-linolenic acid is a minor component of animal phospholipids.
Depending on the cell type, it is the precursor of prostaglandin of 1-series (PGE1), in a reaction catalyzed by ciclooxygenase 1 (COX-1), and/or is metabolized by the 15-lipoxygenase (15-LOX) into 15-(S)-hydroxy-8,11,13-eicosatrienoic acid (15-HETrE) (in several cell types, including neutrophils, macrophage/monocytes and epidermal cells).
PGE1 and 15-HETrE exert biological and clinical effects.
PGE1 is associated with:
- retarding platelet aggregation and so, in turn, inhibition of thrombus formation;
- lowering blood pressure;
- inhibition of cancer cell growth;
- in vitro inhibition of vascular smooth muscle cell proliferation, a hallmark of the atherogenic process, because associated with atherosclerotic plaque development.
15-HETrE is capable of:
- suppress inflammation also by inhibiting the formation of proinflammatory arachidonic acid-derived 5-lipoxygenase metabolites, e.g., LTC4 and LTB4;
- suppress cell growth, particularly epithelial cells.
Furthermore, clinical efficacy of DGLA is due to the fact that:
- it competes with arachidonic acid for COX-2, thus reducing the formation of PGE2;
- it is modestly metabolized into arachidonic acid, thus limiting further the production of PGE2;
- it can’t be converted to leukotrienes.
On the other hand, DGLA appear to be related to the risk of development diabetes because of the composition of cholesterol ester: the risk increases when esters are rich in saturated fatty acids, palmitoleic acid, gamma-linolenic acid and dihomo-gamma-linolenic acid and low in linoleic acid.
References
- Akoh C.C. and Min D.B. “Food lipids: chemistry, nutrition, and biotechnology” 3th ed. 2008
- Chow Ching K. “Fatty acids in foods and their health implication” 3th ed. 2008
- Fan Y.Y. and Chapkin R.S. Importance of dietary gamma-linolenic acid in human health and nutrition. J Nutr 1998;128:1411-1414. doi:10.1093/jn/128.9.1411
- Vessby B., Aro A., Skarfors E., Berglund L., Salminen I., Lithell H. The risk to develop NIDDM is related to the fatty acid composition of the serum cholesterol esters. Diabetes 1994;43:1353-1357. doi:10.2337/diab.43.11.1353