Alpha-amylase (EC 3.2.1.1) is an enzyme that catalyzes the hydrolysis of α-(1→4) glycosidic bonds present on the straight chains of amylose and amylopectin, the two major components of starch granules.[2]
It is widely distributed in animals, plants, fungi, in the Ascomycota and Basidiomycota phyla, bacteria, in the genus Bacillus, and Archaea. Its wide distribution suggests a central role in carbohydrate metabolism.[15]
In humans, alpha-amylase is encoded by two different genes, and is synthesized mainly in the parotid glands and exocrine pancreas.[9] In plants, the alpha-amylase gene family consists of six different members.[11]
In animals, it is involved in the first step of starch digestion, which leads to the release of the disaccharide maltose, the oligosaccharides maltotriose and alpha-limit dextrins, and small amounts of glucose.[5]
Since starch is the main dietary source of carbohydrates, and therefore of glucose and energy, the activity of alpha-amylase may influence postprandial blood glucose levels.[8]
Alpha-amylase is used in different industrial sectors, as well as in clinical diagnostics.[7]
In plants, and particularly in grasses such as wheat, barley and rice, it is crucial for seed germination and grain maturation.[11][17]
Contents
- History
- Human alpha-amylase genes
- Activity of alpha-amylase
- Salivary alpha-amylase
- Pancreatic alpha-amylase
- Blood sugar control
- Amylase activity in clinical biochemistry
- Wheat alpha-amylase
- References
History
The first detection of an amylase activity dates back to 1811, by the Russian chemist Konstantin Sigizmundovich Kirchhoff, who, working with wheat, discovered an enzymatic activity able to broke down starch.[16]
In 1831, the German chemist Erhard Friedrich Leuchs observed the degradation of starch when mixed with human saliva. Leuchs named ptyalin the agent responsible for such degradation. A few years later, in 1833, Anselme Payen and Jean-François Persoz, French chemists who worked in a sugar factory, isolated from barley an enzyme able to degrade starch, and named it diastase.[5]
The enzyme was named alpha-amylase by Richard Kuhn, in 1925, since he discovered that the hydrolysis products were in the alpha configuration.[13]
In 1894, alpha-amylase was the first enzyme to be produced industrially, extracted from a fungal source, and used to treat digestive disorders. Later, starting from 1917, it was also extracted from Bacillus subtilis and Bacillus mesentericus. Currently, it is mainly produced by genetically modified organisms.[15]
Alpha-amylase, and more generally amylases, of which two other types are known, beta-amylase (EC 3.2.1.2) e la gamma-amylase (EC 3.2.1.3), are widely utilized in different industrial sectors, such as textiles, detergent, feed, food, the production of beer and whiskey, as well as cosmetics, pharmaceuticals, and clinical diagnostics.[7]
Alpha-amylases can be classified, based on the degree of hydrolysis of the substrate, as liquifying, if they cleave 30-40 percent of starch glycosidic bonds, and saccharifying, if they cleave 50-60 percent of the bonds.[15]
Human alpha-amylase genes
In many mammals, including humans, alpha-amylase is encoded by two distinct but closely related genes, present on the short arm of chromosome 1, and named:
- AMY1, which encodes for the protein present in saliva, in mammary gland and in some tumors, such as lung tumor;[5]
- AMY2, which encodes for the enzyme produced by the pancreas. AMY2 is present in two forms, AMY2A and AMY2B, whose copy number differs in different populations, although two copies per gene seems to be a frequent pattern. It also appears that copy numbers of AMY2 may be associated with those of AMY1.[9]
Activity of alpha-amylase
Alpha-amylase catalyze the first step in starch digestion, namely, the hydrolysis of α-(1→4) glycosidic bonds within the linear chains of amylose and amylopectin, acting at random location along the α-glucans. The enzyme is therefore an endoglycosidases, has an optimal pH of about 7, requires the presence of calcium ions, and leads to the release of disaccharides and oligosaccharides in alpha configuration, in particular, maltose and maltotriose from amylose, and maltose, alpha-limiting dextrins and, in small amount, glucose from amylopectin.[2]
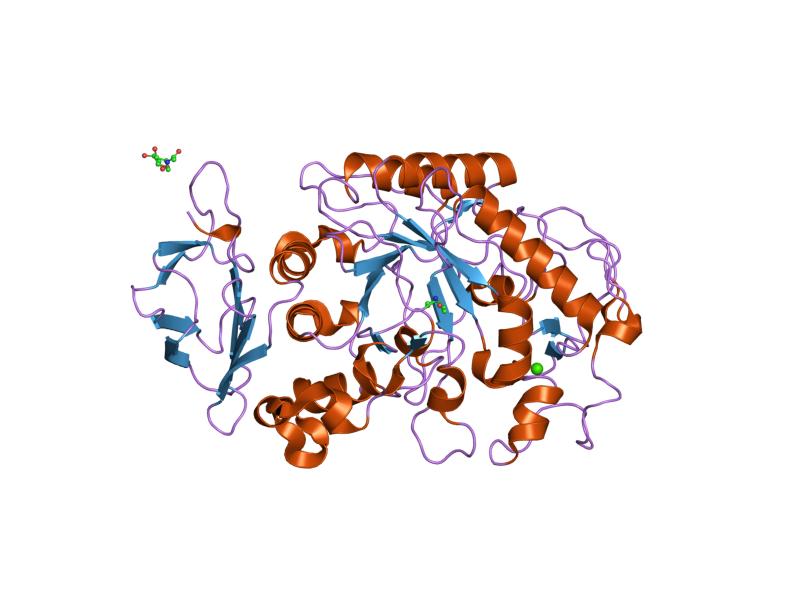
Alpha-amylase, like other enzymes involved in starch metabolism such as pullulanase (EC 3.2.1.41), isoamylase (EC 3.2.1.68), starch branching enzymes (EC 2.4.1.18), and starch debranching enzymes, belongs to the glycoside hydrolase families 13 or GH-13 families, enzymes capable of acting on substrates containing alpha-glycosidic bonds.[10] Two enzymes involved in trehalose metabolism also belong to this family, trehalose synthase (EC 5.4.99.16), which catalyzes the interconversion of maltose and trehalose, and trehalose 6-phosphate hydrolase (EC 3.2.1.93), which hydrolyses trehalose 6-phosphate to yield a molecule of glucose and a molecule of glucose 6-phosphate.
Salivary alpha-amylase
Salivary alpha-amylase or ptyalin is mostly synthesized by the parotid glands, and to a lesser extent by submandibular glands. It is present in a glycosylated- and non-glycosylated form, and is involved in the first, limited, stage of starch digestion in the oral cavity. The enzyme is one of the most abundant salivary proteins, accounting for 40-50 percent of the total salivary proteins, and, with lingual lipase (EC 3.1.1.3), which is involved in lipid digestion, such as digestion of triglycerides and cholesterol esters, is one of the most important salivary enzymes.[3]
Pancreatic alpha-amylase
Pancreatic alpha-amylase is synthesized by the acinar cells of the pancreas.[12] It is one of the major proteins of the pancreatic juice, and, once in the duodenum, it attacks amylose and amylopectin, completing the first stage of the amylolysis. The amylolytic products are then attacked by the alpha-glucosidases of enterocyte brush border, namely, sucrase-isomaltase (EC 3.2.1.48) and maltase-glucoamylase (EC 3.2.1.20), which are also able to hydrolyze the α-(1→2) glycosidic bond of sucrose.[14]
Mammalian pancreatic alpha-amylase is able to bind to N-linked glycans of brush border membrane glycoproteins.[1] Such bond allows the cooperation with sucrase-isomaltase, cooperation that leads to a high increase in glucose production, up to 240 percent. However, it has been observed that glucose uptake by SGLT1, an intestinal transporter involved in the absorption of monosaccharides, is inhibited at high alpha-amylase concentrations in the intestinal lumen. It has been hypothesized that this inhibition may represent an innate regulatory mechanism to avoid sudden increases in glucose absorption and thus facilitate the regulation of postprandial blood glucose levels.[6]
Blood sugar control
Postprandial glycemic control is considered an effective strategy in preventing the development of type II diabetes. Among non-pharmacological methods there is the reduction of the ability of alpha-amylase to attack starch through a food processing leading to starch retrogradation, or by means of molecules present in foods which, with different modes of action, are able to reduce its activity.[8]
Food processing
Starch rich foods are subjected to hydrothermal treatments, such as cooking in boiling water, that cause starch granule gelatinization, namely, they absorb water, swell and lose most of their crystalline structures, which is replaced by amorphous structures, more easily attacked by alpha-amylases.[4] If gelatinized starch is allowed to cool, recrystallization can occur leading to give what is known as retrograded starch, a type of starch resistant to the action of alpha-amylase, which reaches the colon practically intact where it is fermented by the bacteria of the gut microbiota, like soluble fiber.
Role of polyphenols
The digestion of starch by alpha-amylase is affected by molecules present in foods, such as non-starch cell wall polysaccharides, proteins, lipids, and, among phytochemicals, various types of polyphenols.[4] These compounds can limit the action of alpha-amylase in at least four ways.[8]
- Proteins and non-starch polysaccharides are able to limit the loss of the ordered structure of native starch during the hydrothermal processes to which the food is subjected during processing;
- Lipids, polyphenols, proteins and non-starch polysaccharides are able to form ordered structures with starch.
- Proteins, non-starch polysaccharides and polyphenols are able to form physical barriers that reduce the accessibility of hydrolytic enzymes to alpha-glucan chains.
- Polyphenols and non-starch polysaccharides are able to reduce the activity of alpha-amylases and alpha-glucosidases.
Among polyphenols, flavonoids such as proanthocyanidins or condensed tannins seem to be important.[8] In order to be effective, the intake of foods rich in polyphenols, such as black tea or green tea, must be associated with a meal rich in carbohydrates. Gluten seems able to hinder the interaction between polyphenols and starch granules, and therefore reduce their inhibitory effect.
Amylase activity in clinical biochemistry
The assay of serum alpha-amylase activity is used diagnostic laboratory test.[7]
Increased serum levels of amylase activity may result from:
- acute pancreatitis or a flare-up of chronic pancreatitis;
- inflammation of the parotid glands;
- obstruction of the secretory ducts of the parotid glands or the pancreatic duct.
Assays currently in use do not distinguish between salivary and pancreatic isoforms. However, if the increase in amylase activity is associated with an increase in lipase activity, the underlying pathology is probably of pancreatic origin.
Wheat alpha-amylase
In plants, and in particular in wheat, alpha-amylase plays a role in the germination and malting, which is a controlled germination that leads to the conversion of the grain into malt, which is also used for the production of whiskey and beer.[11]
In wheat grains, the enzyme is mainly synthesized in the early stages of germination, which are heterotrophic as they rely heavily on stored resources, and therefore also on the starch present in the endosperm.[17] The synthesis occurs in the aleurone layer, and initiates the mobilization of starch reserves.
Alpha-amylase is also involved in one of the main defects of wheat, pre-harvest germination.
References
- ^ Asanuma-Date K., Hirano Y., Le N., Sano K., Kawasaki N., Hashii N., Hiruta Y., Nakayama K., Umemura M., Ishikawa K., Sakagami H., and Ogawa H. Functional regulation of sugar assimilation by N-glycan-specific interaction of pancreatic α-amylase with glycoproteins of duodenal brush border membrane. J Biol Chem 2012;287(27):23104-18. doi:10.1074/jbc.m111.314658
- ^ a b Benjamin S., Smitha R.B., Jisha V.N., Pradeep S., Sajith S., Sreedevi S., Priji P., Unni K.N., Sarath Josh M.K. A monograph on amylases from Bacillus spp. Adv biosci biotechnol 2013;4:No.2. doi:10.4236/abb.2013.42032
- ^ Boehlke C., Zierau O., Hannig C. Salivary amylase – The enzyme of unspecialized euryphagous animals. Arch Oral Biol 2015;60(8):1162-76. doi:10.1016/j.archoralbio.2015.05.008
- ^ a b Butterworth P.J., Bajka B.H., Edwards C.H., Warren F.J., Ellis P.R. Enzyme kinetic approach for mechanistic insight and predictions of in vivo starch digestibility and the glycaemic index of foods. Trends Food Sci Technol 2022;120:254-264. doi:10.1016/j.tifs.2021.11.015
- ^ a b c Butterworth P.J., Warren F.J. and Ellis P.R. Human α-amylase and starch digestion: an interesting marriage. Starch/Stärke 2011;63:395-405. doi:10.1002/star.201000150
- ^ Date K., Satoh A., Lida K., Ogawa H. Pancreatic α-amylase controls glucose assimilation by duodenal retrieval through N-glycan-specific binding, endocytosis, and degradation. J Biol Chem 2015;290(28):17439-50. doi:10.1074/jbc.M114.594937
- a b c de Souza P.M., de Oliveira Magalhães P. Application of microbial α-amylase in industry – A review. Braz J Microbiol 2010;41(4):850-61. doi:10.1590/S1517-83822010000400004
- ^ a b c d Gong L., Feng D., Wang T., Ren Y., Liu Y., Wang J. Inhibitors of α-amylase and α-glucosidase: potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci Nutr 2020;8(12):6320-6337. doi:10.1002/fsn3.1987
- ^ a b Iafrate A.J., Feuk L., Rivera M.N., Listewnik M.L., Donahoe P.K., Qi Y., Scherer S.W, Lee C. Detection of large-scale variation in the human genome. Nature Genetics. 2004;36:949–951. doi:10.1038/ng1416
- ^ Janeček Š., Svensson B., MacGregor E.A. α-Amylase: an enzyme specificity found in various families of glycoside hydrolases. Cell Mol Life Sci 2014;71(7):1149-70. doi:10.1007/s00018-013-1388-z
- ^ a b c Ju L., Pan Z., Zhang H., Li Q., Liang J., Deng G., Yu M., Long H. New insights into the origin and evolution of α-amylase genes in green plants. Sci Rep 2019;9(1):4929. doi:10.1038/s41598-019-41420-w
- ^ Keller P.J., Allan B.J. The protein composition of human pancreatic juice. J Biol Chem 196725;242(2):281-7. doi:10.1016/S0021-9258(19)81461-8
- ^ Kuhn R. Mechanism of the action of amylases. Constitution of starch. Justus Liebigs Annalen der Chemie 1925;443:1-71
- ^ Nichols B.L., Avery S., Sen P., Swallow D.M., Hahn D., Sterchi E. The maltase-glucoamylase gene: common ancestry to sucrase-isomaltase with complementary starch digestion activities. Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):1432-7. doi:10.1073/pnas.0237170100
- ^ a b c Tiwari S.P., Srivastava R., Singh C.S., Shukla K., Singh R.K., Singh P., Singh R., Singh N.L., and Sharma R. Amylases: an overview with special reference to alpha amylase. Journal of Global Biosciences 2015;4(SI1):1886-1901.
- ^ Zakowski J.J., Bruns D.E. Biochemistry of human alpha amylase isoenzymes. Crit Rev Clin Lab Sci 1985;21(4):283-322. doi:10.3109/10408368509165786
- ^ a b Zhang Q., Pritchard J., Mieog J., Byrne K., Colgrave M.L., Wang J.R., Ral J.F. Over-expression of a wheat late maturity alpha-amylase type 1 impact on starch properties during grain development and germination. Front Plant Sci 2022;13:811728. doi:10.3389/fpls.2022.811728