The Cori cycle, or glucose-lactate cycle, was discovered by Carl Ferdinand Cori and Gerty Theresa Radnitz, a husband-and-wife team, in the ‘30s and ‘40s of the last century . They demonstrated the existence of a metabolic cooperation between the skeletal muscle working under low oxygen conditions and the liver. This cycle can be summarized as follows:
- the conversion of glucose to lactic acid, or lactate, by anaerobic glycolysis in skeletal muscle cells;
- the diffusion of lactate from muscle cells into the bloodstream, by which it is transported to the liver;
- the conversion of lactate to glucose by hepatic gluconeogenesis;
- the diffusion of glucose from the hepatocytes into the bloodstream, by which it is transported back to the skeletal muscle cells, thereby closing the cycle.
Summarizing, we have: part of the lactate produced in skeletal muscle is converted to glucose in the liver, and transported back to skeletal muscle, thus closing the cycle.
Glucose → Lactate → Glucose
The importance of this cycle is demonstrated by the fact that it may account for about 40% of plasma glucose turnover.
Contents
- Where does it occur?
- Steps of the Cori cycle
- Energy cost
- Is the Cori cycle a futile cycle?
- Cori cycle and glucose-alanine cycle
- References
Where does it occur?
In addition to skeletal muscle, this metabolic cooperation was also demonstrated between other extrahepatic tissues and liver. Indeed, like the glucose-alanine cycle, the glucose-lactate cycle is active between the liver and all those tissues that do not completely oxidize glucose to CO2 and H2O, in which case pyruvate for conversion to lactate or, by transamination, to alanine would lack.
In addition to skeletal muscle cells, examples of cells that continually produce lactic acid are red blood cells, immune cells in the lymph nodules, proliferating cells in the bone marrow, and epithelial cells in the skin.
Notice that skeletal muscle produces lactate even at rest, although at low rate.
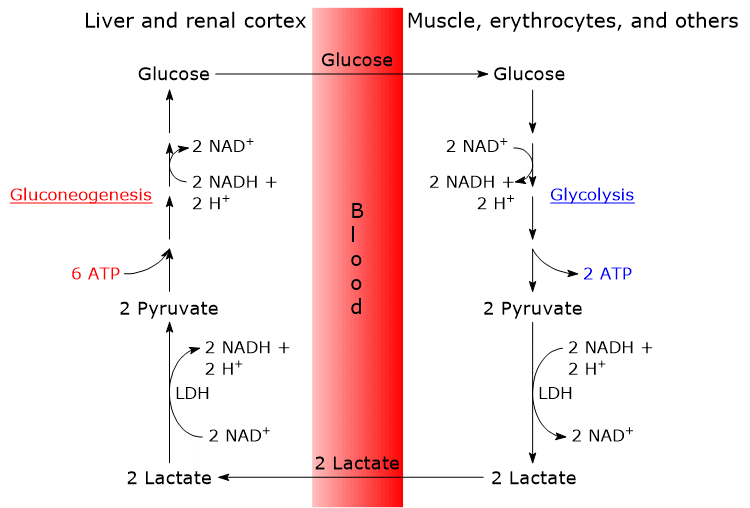
From a biochemical point of view, the Cori cycle links gluconeogenesis with anaerobic glycolysis, using different tissues to compartmentalize opposing metabolic pathways. In fact, in the same cell, regardless of the cell type, these metabolic pathways are not very active simultaneously. Glycolysis is more active when the cell requires ATP; by contrast, when the demand for ATP is low, gluconeogenesis, in those cells where it occurs, is more active.
And it is noteworthy that, although traditionally the metabolic pathways, such as glycolysis, citric acid cycle, or gluconeogenesis, are considered to be confined within individual cells, the Cori cycle, as well as the glucose-alanine cycle, occurs between different cell types.
Finally, it should be underscored that the Cori cycle also involves the renal cortex, particularly the proximal tubules, another site where gluconeogenesis occurs.
Steps of the Cori cycle
The analysis of the steps of the Cori cycle is made considering the lactate produced by red blood cells and skeletal muscle cells.
Mature red blood cells are devoid of mitochondria, nucleus and ribosomes, and obtain the necessary energy only by glycolysis. The availability of NAD+ is essential for glycolysis to proceed as well as for its rate: the oxidized form of the coenzyme is required for the oxidation of glyceraldehyde 3-phosphate to 1,3-bisphosphoglycerate in the reaction catalyzed by glyceraldehyde 3-phosphate dehydrogenase (EC 1.2.1.12).
Glyceraldehyde 3-phosphate + NAD+ → 1,3-Bisphosphoglycerate + NADH + H+
The accumulation of NADH is avoided by the reduction of pyruvate to lactate, in the reaction catalyzed by lactate dehydrogenase (EC 1.1.1.27), where NADH acts as reducing agent.
Pyruvate + NADH + H+ → Lactate + NAD+
The skeletal muscle, particularly fast-twitch fibers which contain a reduced number of mitochondria, under low oxygen condition, such as during intense exercise, produces significant amounts of lactate. In fact, in such conditions:
- the rate of pyruvate production by glycolysis exceeds the rate of its oxidation by the citric acid cycle, so that less than 10% of the pyruvate enters the citric acid cycle;
- the rate at which oxygen is taken up by the cells is not sufficient to allow aerobic oxidation of all the NADH produced.
And, like in red blood cells, the reaction catalyzed by lactate dehydrogenase, regenerating NAD+, allows glycolysis to proceed.
However, lactate is an end product of metabolism that must be converted back into pyruvate to be used.
The plasma membrane of most cells is freely permeable to both pyruvate and lactate that can thus reach the bloodstream. And, regarding for example the skeletal muscle, the amount of lactate that leaves the cell is greater than that of pyruvate due to the high NADH/NAD+ ratio in the cytosol and to the catalytic properties of the skeletal muscle isoenzyme of LDH.
Once into the bloodstream, lactate reaches the liver, which is its major user, where it is oxidized to pyruvate in the reaction catalyzed by the liver isoenzyme of lactate dehydrogenase.
Lactate + NAD+ → Pyruvate + NADH + H+
In the hepatocyte, this oxidation is favored by the low NADH/NAD+ ratio in the cytosol.
Then, pyruvate enters the gluconeogenesis pathway to be converted into glucose.
Glucose leaves the liver, enters into the bloodstream and is delivered to the muscle, as well as to other tissues and cells that require it, such as red blood cells and neurons, thus closing the cycle.
Lactate dehydrogenase
The enzyme is a tetramer composed of two different types of subunits, designed as:
- H subunit (heart) or B chain;
- M subunit (muscle) or A chain.
The H subunit predominates in the heart, whereas the M subunit predominates in the skeletal muscle and liver. Typically, tissues in which a predominantly or exclusively aerobic metabolism occurs, such as the heart, synthesize H subunits to a greater extent than M subunits, whereas tissues in which anaerobic metabolism is important, such as skeletal muscle, synthesize M subunits to a greater extent than H subunits.
The two subunits associate in 5 different ways to form homopolymers, that is, macromolecules formed by repeated, identical subunits, or heteropolymers, that is, macromolecules formed by different subunits. Different LDH isoenzymes have different catalytic properties, as well as different distribution in various tissues, as indicated below:
- H4, also called type 1, LDH1, or A4, a homopolymer of H subunits, is found in cardiac muscle, kidney, and red blood cells;
- H3M1, also called type 2, LDH2, or A3B, has a tissue distribution similar to that of LDH1;
- H2M2, also called type 3, LDH3, or A2B2, is found in the spleen, brain, white cells, kidney, and lung;
- H1M3, also called type 4, LDH4, or AB3, is found in the spleen, lung, skeletal muscle, lung, red blood cells, and kidney;
- M4, also called type 5, LDH5, or B4, a homopolymer of M subunits, is found in the liver, skeletal muscle, and spleen.
The H4 isoenzyme has a higher substrate affinity than the M4 isoenzyme, and is allosterically inhibited by high levels of pyruvate, its product, whereas the M4 isoenzyme is not.
The other LDH isoenzymes have intermediate properties, depending on the ratio between the two types of subunits.
It is thought that the H4 isoenzyme is the most suitable for catalyzing the oxidation of lactate to pyruvate that, in the heart, due to its exclusively aerobic metabolism, is then completely oxidized to CO2 and H2O. Instead, the M4 isoenzyme is the main isoenzyme found in skeletal muscle, most suitable for catalyzing the reduction of pyruvate to lactate, thus allowing glycolysis to proceed in anaerobic conditions.
Other metabolic fates of lactate
From the above, it is clear that lactate is not a metabolic dead end, a waste product of glucose metabolism.
And it may have a different fate from that entering the Cori cycle.
For example, in skeletal muscle during recovery following an exhaustive exercise, that is, when oxygen is again available, or if the exercise is of low intensity, lactate is re-oxidized to pyruvate, due to NAD+ availability, and then completely oxidized to CO2 and H20, with a greater production of ATP than in anaerobic condition. In such conditions, the energy stored in NADH will be released, yielding on average 2.5 ATP per molecule of NADH.
In addition, lactate can be taken up by exclusively aerobic tissues, such as heart, to be oxidized to CO2 and H20.
Energy cost
The Cori cycle results in a net consumption of 4 ATP.
The gluconeogenic leg of the cycle consumes 2 GTP and 4 ATP per molecule of glucose synthesized, that is, 6 ATP.
ATP-consuming reactions are catalyzed by:
- pyruvate carboxylase (EC 6.4.1.1): one ATP;
- phosphoenolpyruvate carboxykinase (EC 4.1.1.32): one GTP;
- glyceraldehyde 3-phosphate dehydrogenase (EC 1.2.1.12): one ATP.
Since two molecules of lactate are required for the synthesis of one molecule of glucose, the net cost is 2 x 3 = 6 high energy bonds per molecule of glucose.
Conversely, the glycolytic leg of the cycle produces only 2 ATP per molecule of glucose.
Therefore, more energy is required to produce glucose from lactate than that obtained by anaerobic glycolysis in extrahepatic tissues. This explains why the Cori cycle cannot be sustained indefinitely.
Is the Cori cycle a futile cycle?
The continuous breakdown and resynthesis of glucose, feature of the Cori cycle, might seem like a waste of energy. Indeed, this cycle allows the effective functioning of many extrahepatic cells at the expense of the liver and partly of the renal cortex. Therefore, it is therefore more correct to define it as a substrate cycle rather than a futile cycle. Below, two examples.
- Red blood cells
These cells, lacking a nucleus, ribosomes, and mitochondria, are smaller than most other cells. Their small size allows them to pass through tiny capillaries. However, the lack of mitochondria makes them completely dependent on anaerobic glycolysis for ATP production. Then, the lactate is partly disposed of by the liver and renal cortex. - Skeletal muscle
Its cells, and particularly fast-twitch fibers contracting under low oxygen conditions, such as during intense exercise, produce much lactate.
In such conditions, anaerobic glycolysis leads to the production of 2 ATP per molecule of glucose, 3 if the glucose comes from muscle glycogen, therefore, much lower than the 29-30 ATP produced by the complete oxidation of the monosaccharide. However, the rate of ATP production by anaerobic glycolysis is greater than that produced by the complete oxidation of glucose. Therefore, to meet the energy requirements of contracting muscle, anaerobic glycolysis is an effective means of ATP production. But this could lead to an intracellular accumulation of lactate, and a consequent reduction in intracellular pH. Obviously, such accumulation does not occur, due also to the Cori cycle, in which the liver pays the cost of the disposal of a large part of the muscle lactate, thereby allowing the muscle to use ATP for the contraction.
And the oxygen debt, which always occurs after a strenuous exercise, is largely due to the increased oxygen demand of the hepatocytes, in which the oxidation of fatty acids, their main fuel, provides the ATP required for gluconeogenesis from lactate. - During trauma, sepsis, burns, or after major surgery, an intense cell proliferation occurs in the wound, that is a hypoxic tissue, and in bone marrow. This in turn results in greater production of lactate, an increase in the flux through the Cori cycle and an increase in ATP consumption in the liver, which, as previously said, is supported by an increase in the oxidation of fatty acids. Hence, the nutrition plan provided to these patients must be taken into account this increase in energy consumption.
- A similar condition seems to occur also in cancer patients with progressive weight loss.
- The Cori cycle is also important during overnight fasting and starvation.
Cori cycle and glucose-alanine cycle
These cycles are metabolic pathways that contribute to ensure a continuous delivery of glucose to tissues for which the monosaccharide is the primary source of energy.
The main difference between the two cycles consists in the three carbon intermediate which is recycled: in the Cori cycle, carbon returns to the liver in the form of pyruvate, whereas in the glucose-alanine cycle in the form of alanine.
For more information, see: glucose-alanine cycle.
References
- American Chemical Society National Historic Chemical Landmarks. Carl and Gerty Cori and Carbohydrate Metabolism. https://www.acs.org/education/whatischemistry/landmarks/carbohydratemetabolism.html
- Bender D.A. Introduction to nutrition and metabolism. 3rd Edition. Taylor & Francis, 2004
- Iqbal S.A., Mido Y. Biochemistry. Discovery Publishing House, 2005
- Nelson D.L., M. M. Cox M.M. Lehninger. Principles of biochemistry. 6th Edition. W.H. Freeman and Company, 2012
- Newsholme E.A., Leech T.R. Functional biochemistry in health and disease. John Wiley J. & Sons, Inc., Publication, 2010
- Rosenthal M.D., Glew R.H. Medical biochemistry – Human metabolism in health and disease. John Wiley J. & Sons, Inc., Publication, 2009
- Stipanuk M.H., Caudill M.A. Biochemical, physiological, and molecular aspects of human nutrition. 3rd Edition. Elsevier health sciences, 2012