The human gastrointestinal tract is one of the most fierce and competitive ecological niches. It harbors viruses, eukaryotes, bacteria, and one member of Archaebacteria, Methanobrevibacter smithii.
Bacteria vary in proportion and amount all along the gastrointestinal tract; the greatest amount is found in the colon, which contains over 400 different species belonging to 9 phyla or divisions, of the 30 recognized phyla, hereafter referred as gut microbiota, which in turn is part of the larger human microbiota.
These are the phyla and some of their most represented genera.
- Actinobacteria, Gram-positive bacteria; Bifidobacterium, Collinsella, Eggerthella, and Propionibacterium.
- Bacteroidetes, Gram-negative bacteria; more than 20 genera including Bacteroides, Prevotella and Corynebacterium.
- Cyanobacteria, Gram-negative bacteria.
- Firmicutes, Gram-positive bacteria; at least 250 genera, including Mycoplasma, Bacillus, Clostridium, Dorea, Faecalibacterium, Ruminococcus, Eubacterium, Staphylococcus, Streptococcus, Lactobacillus, Lactococcus, Enterococcus, Sporobacter, and Roseburia.
- Fusobacteria, Gram-negative bacteria.
- Lentisphaerae, Gram-negative bacteria.
- Proteobacteria, Gram-negative bacteria; Escherichia, Klebsiella, Shigella, Salmonella, Citrobacter, Helicobacter, and Serratia.
- Spirochaeates, Gram-negative bacteria.
- Verrucomicrobia, Gram-negative bacteria.
The presence of a small subset of the bacterial world in the colon is the result of a strong selective pressure which acted, during evolution, on both the microbial colonizers, selecting organisms very well adapted to this environment, and the intestinal niche. And nevertheless, each individual harbors an unique bacterial community in his gut.
Despite the high variability existing both with regard to taxa and between individuals, it has been proposed, but not accepted by all researchers, that in most adults the bacterial gut microbiota can be classified into variants or “enterotypes”, on the basis of the ratio of the abundance of the genera Bacteroides and Prevotella. This seems to indicate that there is a limited number of well balanced symbiotic states, which could respond differently to factors such as diet, age, genetics, and drug intake.
Adult’s gut harbors a large and diverse community of DNA and RNA viruses made up of about 2,000 different genotypes, none of which is dominant. Indeed, the most abundant virus accounts for only about 6 percent of the community, whereas in infants the most abundant virus accounts over 40 percent of the community. The majority of DNA viruses are bacteriophages or phages, that is, viruses that infect bacteria. They are the most abundant biological entity on earth, with an estimated population of about 1031 units, whereas the majority of RNA viruses are plant viruses.
Contents
Factors affecting gut microbiota composition and development
The intestinal bacterial community is regulated by several factors, most of which are listed below.
- The diet of the host.
It seems to be the most important factor.
Traditionally considered sterile, mother’s milk harbors a rich microbiota consisting of more than 700 species, dominated by staphylococci, streptococci, bifidobacteria and lactic acid bacteria. Therefore, it is a major source for the colonization of the breastfed infant gut, and it was suggested that this mode of colonization is closely correlated with infant’s health status, because, among other functions, it could protect against infections and contribute to the maturation of the immune system. Breast milk affects intestinal microbiota also indirectly, through the presence of oligosaccharides with prebiotic activity that stimulate the growth of specific bacterial groups including staphylococci and bifidobacteria.
A recent study has compared the intestinal microbiota of European and African children, respectively from Florence and a rural village in Burkina Faso, between the ages of 1 and 6 years old. It has highlighted the dominant role of diet over variables such as climate, geography, hygiene and health services; it was also observed the absence of significant differences in the expression of key genes regulating the immune function, which suggests a functional similarity between the two groups. Indeed infants, as long as they are breastfed, have a very similar gut microbiota, rich in Actinobacteria, mainly Bifidobacterium.
The subsequent introduction of solid foods in the two groups, a Western diet rich in animal fats and proteins in European children, and low in animal proteins but rich in complex carbohydrates in African children, leads to a differentiation in the Firmicutes/Bacteroidetes ratio between the two groups. Gram-positive bacteria, mainly Firmicutes, were more abundant than Gram-negative bacteria in European children, whereas Gram-negative bacteria, mainly Bacteroidetes, prevailed over Gram-positive bacteria in African children.
And the long-term diets are strongly associated to the enterotype partitioning. Indeed, it has been observed that:
a diet high in animal fats and proteins, i.e. a Western-type diet, leads to a gut microbiota dominated by the Bacteroides enterotype;
a diet high in complex carbohydrates, typical of agrarian societies, leads to the prevalence of the Prevotella enterotype.
Similar results emerged from the aforementioned study on children. In the Europeans, gut microbiota was dominated by taxa typical of Bacteroides enterotype, whereas in the Burkina Faso children, Prevotella enterotype dominates.
With short-term changes in the diet, 10 days, such as the switch from a low-fat and high-fiber diet to a high-fat and low-fiber diet and vice versa, changes were observed in the composition of the microbiome, within 24 hours, but no stable change in the enterotype partitioning. And this underlines as a long-term diet is needed for a change in the enterotypes of the gut microbiota.
Dietary interventions can also result in changes in the gut virome, which moves to a new state, that is, changes occur in the proportions of the pre-existing viral populations, towards which subjects on the same diet converge.
- pH, bile salts and digestive enzymes.
The stomach, due to its low pH, is a hostile environment for bacteria, which are not present in high numbers, about 102-103 bacterial cells/gram of tissue. In addition to Helicobacter pylori, able to cause gastritis and gastric ulcers, microorganisms of the genus Lactobacillus are also present.
Reached the duodenum, an increase in bacterial cell number occurs, 104-105 bacterial cells/gram of tissue; and similar bacterial concentrations are present in the jejunum and proximal ileum. The low number of microorganisms present in the small intestine is due to the inhospitable environment, consequent to the fact that there is the opening of the ampulla of Vater in the descending part of the duodenum, which pours pancreatic juice and bile into the duodenum, that is, pancreatic enzymes and bile salts, which damage microorganisms.
In the terminal portion of the ileum, where the activities of pancreatic enzymes and bile salts are lower, there are about 107 bacterial cells/gram of tissue, and up to 1012-1014 bacterial cells/gram of tissue in the colon, so that bacteria represent a large proportion, about 40 percent, of the fecal mass.
The distribution of bacteria along the intestine is strategic. In the duodenum and jejunum, the amount of available nutrients is much higher than that found in the terminal portion of the ileum, where just water, fiber, and electrolytes remain. Therefore, the presence of large number of bacteria in the terminal portion of the ileum, and even more in the colon, is not a problem. The problem would be to find a high bacterial concentration in the duodenum, jejunum, and proximal parts of the ileum; and there is a disease condition, called small intestinal bacterial overgrowth or SIBO, in which the number of bacteria in the small intestine increases by about 10-15 times. This puts them in a position to compete with the host for nutrients and give rise to gastrointestinal disturbances such as diarrhea. - The geographical position and the resulting differences in lifestyle, diet, religion etc.
For example, a kind of geographical gradient occurs in the microbiota of European infants, with a higher number of Bifidobacterium species and some of Clostridium in Northern infants, whereas Southern infants have higher levels of Bacteroides, Lactobacillus and Eubacterium. - The mode of delivery.
- The genetics of the host.
- The health status of the infant and mother.
For example, in mothers with inflammatory bowel disease or IBD, Faecalibacterium prausnitzii, a bacterium that produces butyric acid, an important source of energy for intestinal cells, and with anti-inflammatory activity is depleted, whereas there is an increase in the number of adherent Escherichia coli. - The treatment with antibiotics.
- Bacterial infections and predators.
Bacteriocins, i.e. proteins with antibacterial activity, and bacteriophages.
Phages play an important role in controlling the abundance and composition of the gut microbiota. In particular, they could play a major role in the colonization of the newborn, infecting the dominant bacteria thus allowing to another bacterial strain to become abundant.
This model of predator-prey dynamics, called “kill the winner”, suggests that the blooms of a specific bacterial species would lead to blooms of their corresponding bacteriophages, followed by a decline in their abundance. Therefore, the most abundant bacteriophage genotype will not be the same at different times. And although some the gene sequences present in the infant gut virome are stable over the first three months of life, dramatic changes occur in the overall composition of the viral community between the first and second week of life. During this time period also the bacterial community is extremely dynamic. - The competition for space and nutrients.
Composition throughout life
The development of the intestinal microbial ecosystem is a complex and crucial event in human life, highly variable from individual to individual, and influenced by the factors outlined above.
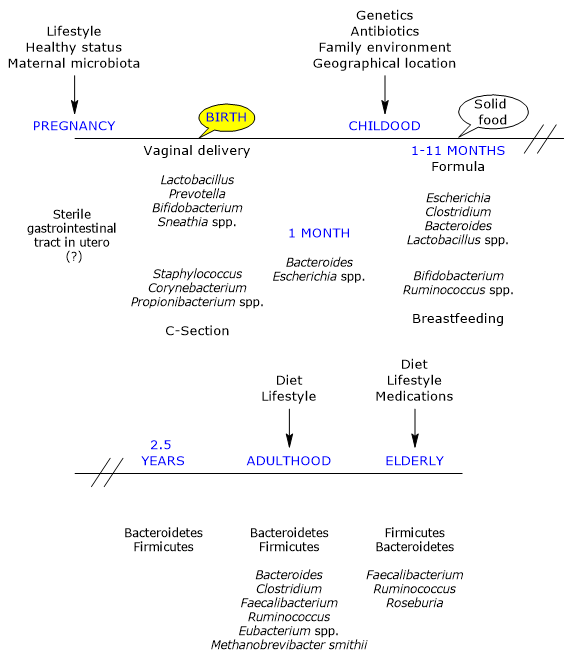
In utero, the gut is considered sterile, but is rapidly colonized by microbes at birth, as the infant is born with an immunological tolerance instructed by the mother.
However, recent studies show the presence of bacteria in the placental tissue, umbilical cord blood, fetal membranes and amniotic fluid from healthy newborns without signs of infection or inflammation. And for example, the meconium of premature infants, born to healthy mothers, contains a specific microbiota, with Firmicutes as the main phylum, and predominance of staphylococci, whereas Proteobacteria, in particular species such as Escherichia coli, Klebsiella pneumoniae, Serratia marcescens, but also enterococci are more abundant in the faeces.
Note: The meconium is free of detectable viruses.
It seems that both vaginal and gut bacteria may gain access to the fetus, although via different route of entry: by ascending entry the vaginal ones, by dendritic cells of the immune system the gut ones. Therefore, there could exist a fetal microbiota.
Colonization occurs during delivery by a maternal inoculum, generally composed of aerobic and facultative bacteria (the newborn’s gut initially contains oxygen), then replaced by obligate anaerobes, bacteria typically present in adulthood, to which they have created a hospitable environment.
Furthermore, there is a small number of different taxa, with a relative dominance of the phyla Actinobacteria and Proteobacteria, that remains unchanged during the first month of life, but not in the subsequent ones as there is a large increase in variability and new genetic variants. Many studies underline that the initial exposure is important in defining the “trajectories” which will lead to the adult ecosystems. Additionally, these initial communities may act as a source of protective or pathogenic microorganisms.
Mother’s vaginal and fecal microbiotas are the main sources of inoculum in vaginally delivered infants. Indeed, infants harbor microbial communities dominated by species of the genera Lactobacillus, the most abundant genus in the vaginal microbiota and early gut microbiota, Bifidobacterium, Prevotella, or Sneathia. And it seems likely that anaerobes, such as members of the phyla Firmicutes and Bacteroidetes, not growing outside of their host, rely on the close contact between mother and offspring for transmission. Finally, due to the presence of oxygen in infant gut, the transmission of strict anaerobes could occur not directly at birth but at a later stage by means of spores.
The first bacteria encountered by infants born by caesarean section are those of the skin and hospital environment, and gut microbiota is dominated by species of the genera Corynebacterium, Staphylococcus and Propionibacterium, with a lower bacterial count and diversity in first weeks of life than infants born vaginally.
Further evidence supporting the hypothesis of vertical transmission is the similarity between the microbiota of meconium and samples obtained from possible sites of contamination.
These “maternal bacteria” do not persist indefinitely, and are replaced by other populations within the first year of life.
Objects, animals, mouths and skin of relatives, and breast milk are secondary sources of inoculum; and breast milk seems to have a primary role in determining the microbial succession in the gut.
The variation and diversity among children reflect instead the individuality of these microbial exposures.
Note: The delivery mode seems also to influence the immune system during the first year of life, perhaps via the influence on the development of gut microbiota. Infants born by cesarean section have:
- a lower bacterial count in stool samples at one month of age, mainly due to the higher number of bifidobacteria in infants born vaginally;
- a higher number of antibody secreting cells, which could reflect an excessive antigen exposure (the intestinal barrier would be more vulnerable to the passage of antigens).
Within a days after birth, a thriving community is established. This community is less stable over time and more variable in composition than that of adults. Very soon, it will be more numerous than that of the child’s cells, evolving according to a temporal pattern highly variable from individual to individual.
Viruses, absent at birth, reach about 108 units/gram wet weight of faeces by the end of the first week of life, therefore representing a dynamic and abundant component of the developing gut microbiota. However, viral community has an extremely low diversity, like bacteria, and is dominated by phages, which probably influence the abundance and diversity of co-occurring bacteria, as seen above. The initial source of the viruses is unknown; of course, maternal and/or environmental inocula are among the possibilities. Notably, the earliest viruses could be the result of induction of prophages from the “newborn” gut bacterial flora, hypothesis supported by the observation that more than 25 percent of the phage sequences seem to be very similar to those of phages infecting bacteria such as Lactococcus, Lactobacillus, Enterococcus, and Streptococcus, which are abundant in breast milk.
By the end of the first month of life it is thought that the initial phase of rapid acquisition of microorganism is over.
In 1-month-old-infants, the most abundant bacteria belong to the genera Bacteroides and Escherichia, whereas Bifidobacterium, along with Ruminococcus, appear and grow to become dominant in the gastrointestinal tract of the breastfed infants between 1 and 11 months. Bifidobacteria such as Bifidobacterium longum subspecies infantis:
- are known to be closely related to breastfeeding;
- are among the best characterized commensal bacteria;
- are considered probiotics, that is, microorganisms which can confer health benefits to the host.
Their abundance confers also benefits through competitive exclusion, that is, they are an obstacle to colonization by pathogens. And indeed, Escherichia and Bacteroides can become preponderant if Bifidobacterium is not adequately present in the gut.
In contrast, bacteria of the genera Escherichia, such as E. coli), Clostridium, such as C. difficile, Bacteroides, such as B. fragilis, and Lactobacillus are present in higher levels in formula-fed infants than in breastfed infants.
Although breast-fed infants receive only breast milk until weaning, their microbiota can show a large variability in the abundances of bacterial taxa, with differences between individuals also with regard to the temporal patterns of variation. These variations may be due to diseases, treatments with antibiotics, changes in host lifestyle, random colonization events, as well as differences in immune responses to the gut colonizing microbes. However, it is not yet clear how these factors contribute to shape infant gut microbiota.
It seems that also the virome changes rapidly after birth, as the majority of the viral sequences present in the first week of life are not found after the second week. Moreover, the repertoire expands rapidly in number and diversity during the first three months. This is in contrast with the stability observed in the adult virome, where 95% of the sequences are conserved over time.
In normal condition, towards the end of the first year of life, babies have consumed an adult-like diet for a significant time period and should have developed a microbial community with characteristics similar to those found in the adult gut, such as:
- a more stable composition, phylogenetically more complex, and progressively more similar among different subjects;
- a preponderance of Firmicutes and Bacteroidetes, followed by Verrucomicrobia and a very low abundance of Proteobacteria;
- an increase in the levels of short-chain fatty acids, mainly acetic acid, propionic acid and butyric acid, and bacterial load in the feces;
- an increase of genes associated with xenobiotic degradation, vitamin biosynthesis, and carbohydrate utilization.
Interestingly, the significant turnover of taxa occurring from birth to the end of the first year is accompanied by a remarkable constancy in the overall functional capabilities.
Towards the end of the first year of life also the early viral colonizers were replaced by a community specific to the child.
The gut microbiota reaches maturity at about 2.5 years of age, fully resembling the adult gut microbiota.
The selection of the most adapted bacteria is the result of various factors.
- The transition to an adult diet.
- An increased fitness to the intestinal environment of the taxa that typically dominate the adult gut microbiota than the early colonizers.
- The significant changes in the intestinal environment, result of the developmental changes in the intestinal mucosa.
- The effects of the microbiota itself.
Therefore, the first 2-3 years of life are the most critical period in which you can intervene to shape the microbiota as best as possible, and so optimize child growth and development.
From a chaotic beginning, all this leads to the establishment of the gut ecosystem typical of the young adult, which is relatively stable over time until old age, viral, archaeal and eukaryotic components included, and dominated, at least in the western population, by members of the phyla Firmicutes, about 60% of the bacterial communities, Bacteroidetes and Actinobacteria, mainly belonging to the Bifidobacterium genus, each comprising about 10 percent of the bacterial community, followed by Proteobacteria and Verrucomicrobia. The genera Bacteroides, Clostridium, Faecalibacterium, Ruminococcus and Eubacterium make up, together with Methanobrevibacter smithii, the large majority of the adult gut microbial community.
It should be noted that different data were obtained from analysis of populations of African rural areas, as seen above.
And the gut microbiota is sufficiently similar among subjects to allow the identification of a shared core microbiome.
Stability and resilience, however, are subject to numerous variables among which, as previously said, diet seems to be one of the most important. Therefore, in order to maintain the stability of the gut microbiota, the variables have to be kept constant, or in the case of diseases prevented, also through vaccinations. However, the stability and resilience could be harmful if the dominant community is pathogenic.
The gut microbiota undergoes substantial changes in the elderly. In a study conducted in Ireland on 161 healthy people aged 65 years and over, the gut microbiota is distinct from that of younger adults in the majority of subjects, with a composition that seems to be dominated by the phyla Bacteroidetes, the main ones, and Firmicutes, with almost inverted percentages than those found in younger adults, although large variations across subjects were observed. And there are Faecalibacterium, about 6 percent of the main genera, followed by species of the genera Ruminococcus, Roseburia and Bifidobacterium ,the latter about 0.4 percent, among the most abundant genera.
Also the variability in the composition of the community is greater than in younger adults; this could be due to the increase in morbidities associated with aging and the subsequent increased intake of medications, as well as to changes in the diet.
References
- Breitbart M., Haynes M., Kelley S., Angly F., Edwards R.A., Felts B., Mahaffy J.M., Mueller J., Nulton J., Rayhawk S., Rodriguez-Brito B., Salamon P., Rohwer F. Viral diversity and dynamics in an infant gut. Res Microbiol 2008;159:367-373. doi:10.1016/j.resmic.2008.04.006
- Claesson M.J., Cusack S., O’Sullivan O., Greene-Diniz R., de Weerd H., Flannery E., Marchesi J.R., Falush D., Dinan T., Fitzgerald G., et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA 2011;108(Suppl 1);4586-4591. doi:10.1073/pnas.1000097107
- Clemente J.C., Ursell L.K., Wegener Parfrey L., and Knight R. The impact of the gut microbiota on human health: an integrative view. Cell 2012;148:1258-1270. doi:10.1016/j.cell.2012.01.035
- De Filippo c., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S., Collini S., Pieraccini G., and Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci 2010;107(33):14691-14696. doi:10.1073/pnas.1005963107
- Dominguez-Bello M.G., Costello E.K., Contreras M., Magris M., Hidalgo G., Fierer N., and Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci 2010;107:11971-11975. doi:10.1073/pnas.1002601107
- Fernández L., Langa S., Martín V., Maldonado A., Jiménez E., Martín R., Rodríguez J.M. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res 2013;69(1):1-10. doi:10.1073/pnas.1002601107
- Huurre A., Kalliomäki M., Rautava S., Rinne M., Salminen S., and Isolauri E. Mode of delivery-effects on gut microbiota and humoral immunity. Neonatology 2008;93:236-240. doi:10.1159/000111102
- Koenig J.E., Spor A., Scalfone N., Fricker A.D., Stombaugh J., Knight R., Angenent L.T., and Ley R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci 2011;108(1):4578-4585. doi:10.1073/pnas.1000081107
- Ley R.E., Peterson D.A., and Gordon J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006;124(4):837-848. doi:10.1016/j.cell.2006.02.017
- Minot S., Sinha R., Chen J., Li H., Keilbaugh S.A., Wu G.D., Lewis J.D., and Bushman F.D. The human gut virome: inter-individual variation and dynamic response to diet. Genome Res 2011;21:1616-1625. doi:10.1101/gr.122705.111
- Moreno-Indias I.M., Cardona F., Tinahones F.J. and Queipo-Ortuño M.I. Impact of the gut microbiota on the development of obesity and type 2 diabetes mellitus. Front Microbiol 2014;5(190):1-10. doi:10.3389/fmicb.2014.00190
- Newburg D.S. & Morelli L. Human milk and infant intestinal mucosal glycans guide succession of the neonatal intestinal microbiota. Pediatr Res 2015;77:115-120. doi:10.1038/pr.2014.178
- Palmer C., Bik E.M., DiGiulio D.B., Relman D.A., and Brown P.O. Development of the human infant intestinal microbiota. PLoS Biol 2007;5(7):e177. doi:10.1371/journal.pbio.0050177
- Rodrıguez J.M., Murphy K., Stanton C., Ross R.P., I. Kober O.I., Juge N., Avershina E., Rudi K., Narbad A., Jenmalm M.C., Marchesi J.R. and Collado M.C. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis 2015;26:26050. doi:10.3402/mehd.v26.26050
- Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.Y., Keilbaugh S.A., Bewtra M., Knights D., Walters W.A., Knight R., et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105-108. doi:10.1126/science.1208344